Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease
- PMID: 20055714
- PMCID: PMC3644209
- DOI: 10.1517/14728220903540257
Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease
Abstract
Importance of the field: Cardiovascular disease is a leading cause of death in modern societies. Hyperpolarizing Ca(2+)-activated K(+) channels (K(Ca)) are important membrane proteins in the control of arterial tone and pathological vascular remodelling and thus could serve as new drug targets.
Areas covered in this review: We summarize recent advances in the field of vascular K(Ca) and their roles in cardiovascular pathologies such as hypertension and restenosis disease and draw attention to novel small-molecule channel modulators and their possible therapeutic utility. This review focuses on literature from the last four to five years.
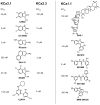
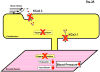
What the reader will gain: Pharmacological opening of endothelial KCa3.1/KCa2.3 channels stimulates endothelium-derived-hyperpolarizing-factor-mediated arteriolar dilation and lowers blood pressure. Inhibition of smooth muscle KCa3.1 channels has beneficial effects in restenosis disease and atherosclerosis. We consider the therapeutic potential of KCa3.1/KCa2.3 openers as novel endothelium-specific antihypertensive drugs as well as of KCa3.1-blockers for the treatment of pathological vascular remodelling and discuss advantages and disadvantages of the pharmacotherapeutic approaches.
Take home message: Pharmacological manipulation of vascular K(Ca) channels by novel small-molecule modulators offers new venues for alternative treatments of hypertension, restenosis and atherosclerosis. Additional efforts are required to optimize these compounds and to validate them as cardiovascular-protective drugs.
Figures


Similar articles
-
Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses--relevance to cardiovascular pathologies and drug discovery.Br J Pharmacol. 2009 Jun;157(4):509-26. doi: 10.1111/j.1476-5381.2009.00132.x. Epub 2009 Mar 19. Br J Pharmacol. 2009. PMID: 19302590 Free PMC article. Review.
-
Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications.Curr Med Chem. 2007;14(13):1437-57. doi: 10.2174/092986707780831186. Curr Med Chem. 2007. PMID: 17584055 Review.
-
Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice.Pflugers Arch. 2010 May;459(6):969-76. doi: 10.1007/s00424-010-0819-z. Epub 2010 Mar 28. Pflugers Arch. 2010. PMID: 20349244 Review.
-
Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure.Mol Pharmacol. 2009 Feb;75(2):281-95. doi: 10.1124/mol.108.051425. Epub 2008 Oct 27. Mol Pharmacol. 2009. PMID: 18955585 Free PMC article.
-
Endothelial Small- and Intermediate-Conductance K Channels and Endothelium-Dependent Hyperpolarization as Drug Targets in Cardiovascular Disease.Adv Pharmacol. 2016;77:65-104. doi: 10.1016/bs.apha.2016.04.002. Epub 2016 May 4. Adv Pharmacol. 2016. PMID: 27451095 Review.
Cited by
-
Pu-Erh Tea Relaxes the Thoracic Aorta of Rats by Reducing Intracellular Calcium.Front Pharmacol. 2019 Nov 28;10:1430. doi: 10.3389/fphar.2019.01430. eCollection 2019. Front Pharmacol. 2019. PMID: 31849675 Free PMC article.
-
Mechanisms underlying selective coupling of endothelial Ca2+ signals with eNOS vs. IK/SK channels in systemic and pulmonary arteries.J Physiol. 2020 Sep;598(17):3577-3596. doi: 10.1113/JP279570. Epub 2020 Jun 11. J Physiol. 2020. PMID: 32463112 Free PMC article.
-
KCa1.1 potassium channels regulate key proinflammatory and invasive properties of fibroblast-like synoviocytes in rheumatoid arthritis.J Biol Chem. 2012 Feb 3;287(6):4014-22. doi: 10.1074/jbc.M111.312264. Epub 2011 Nov 10. J Biol Chem. 2012. PMID: 22074915 Free PMC article.
-
Plasma membrane insertion of KCa2.3 (SK3) is dependent upon the SNARE proteins, syntaxin-4 and SNAP23.PLoS One. 2018 May 16;13(5):e0196717. doi: 10.1371/journal.pone.0196717. eCollection 2018. PLoS One. 2018. PMID: 29768434 Free PMC article.
-
High glucose induces CCL20 in proximal tubular cells via activation of the KCa3.1 channel.PLoS One. 2014 Apr 14;9(4):e95173. doi: 10.1371/journal.pone.0095173. eCollection 2014. PLoS One. 2014. PMID: 24733189 Free PMC article.
References
-
- Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–92. - PubMed
-
- Spieker LE, Flammer AJ, Luscher TF. The vascular endothelium in hypertension. Handb Exp Pharmacol. 2006:249–83. - PubMed
-
- Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. - PubMed
-
- Zargham R. Preventing restenosis after angioplasty: a multistage approach. Clin Sci (Lond) 2008;114:257–64. - PubMed
-
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
