Dynamic causal modelling of effective connectivity from fMRI: are results reproducible and sensitive to Parkinson's disease and its treatment?
- PMID: 20056151
- PMCID: PMC3021391
- DOI: 10.1016/j.neuroimage.2009.12.080
Dynamic causal modelling of effective connectivity from fMRI: are results reproducible and sensitive to Parkinson's disease and its treatment?
Abstract
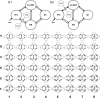
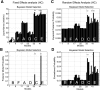
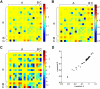
Dynamic causal modelling (DCM) of functional magnetic resonance imaging (fMRI) data offers new insights into the pathophysiology of neurological disease and mechanisms of effective therapies. Current applications can be used both to identify the most likely functional brain network underlying observed data and estimate the networks' connectivity parameters. We examined the reproducibility of DCM in healthy subjects (young 18-48 years, n=27; old 50-80 years, n=15) in the context of action selection. We then examined the effects of Parkinson's disease (50-78 years, Hoehn and Yahr stage 1-2.5, n=16) and dopaminergic therapy. Forty-eight models were compared, for each of 90 sessions from 58 subjects. Model-evidences clustered according to sets of structurally similar models, with high correlations over two sessions in healthy older subjects. The same model was identified as most likely in healthy controls on both sessions and in medicated patients. In this most likely network model, the selection of action was associated with enhanced coupling between prefrontal cortex and the pre-supplementary motor area. However, the parameters for intrinsic connectivity and contextual modulation in this model were poorly correlated across sessions. A different model was identified in patients with Parkinson's disease after medication withdrawal. In "off" patients, action selection was associated with enhanced connectivity from prefrontal to lateral premotor cortex. This accords with independent evidence of a dopamine-dependent functional disconnection of the SMA in Parkinson's disease. Together, these results suggest that DCM model selection is robust and sensitive enough to study clinical populations and their pharmacological treatment. For critical inferences, model selection may be sufficient. However, caution is required when comparing groups or drug effects in terms of the connectivity parameter estimates, if there are significant posterior covariances among parameters.
Copyright (c) 2009 Elsevier Inc. All rights reserved.
Figures



References
-
- Acs F., Greenlee M.W. Connectivity modulation of early visual processing areas during covert and overt tracking tasks. Neuroimage. 2008;41:380–388. - PubMed
-
- Allen P., Mechelli A., Stephan K.E., Day F., Dalton J., Williams S., McGuire P.K. Fronto-temporal interactions during overt verbal initiation and suppression. J. Cogn. Neurosci. 2008;20:1656–1669. - PubMed
-
- Buhmann C., Glauche V., Sturenburg H.J., Oechsner M., Weiller C., Buchel C. Pharmacologically modulated fMRI-cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain. 2003;126:451–461. - PubMed
-
- Deiber M.P., Passingham R.E., Colebatch J.G., Friston K.J., Nixon P.D., Frackowiak R.S. Cortical areas and the selection of movement: a study with positron emission tomography. Exp. Brain Res. 1991;84:393–402. - PubMed
-
- Deneux T., Faugeras O. Using nonlinear models in fMRI data analysis: model selection and activation detection. Neuroimage. 2006;32:1669–1689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

