Engineering filamentous phage carriers to improve focusing of antibody responses against peptides
- PMID: 20056188
- PMCID: PMC2830289
- DOI: 10.1016/j.vaccine.2009.12.059
Engineering filamentous phage carriers to improve focusing of antibody responses against peptides
Abstract
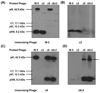
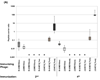
The filamentous bacteriophage are highly immunogenic particles that can be used as carrier proteins for peptides and presumably other haptens and antigens. Our previous work demonstrated that the antibody response was better focused against a synthetic peptide if it was conjugated to phage as compared to the classical carrier, ovalbumin. We speculated that this was due, in part, to the relatively low surface complexity of the phage. Here, we further investigate the phage as an immunogenic carrier, and the effect reducing its surface complexity has on the antibody response against peptides that are either displayed as recombinant fusions to the phage coat or are chemically conjugated to it. Immunodominant regions of the minor coat protein, pIII, were removed from the phage surface by excising its N1 and N2 domains (Delta3 phage variant), whereas immunodominant epitopes of the major coat protein, pVIII, were altered by reducing the charge of its surface-exposed N-terminal residues (Delta8 phage variant). Immunization of mice revealed that the Delta3 variant was less immunogenic than wild-type (WT) phage, whereas the Delta8 variant was more immunogenic. The immunogenicity of two different peptides was tested in the context of the WT and Delta3 phage in two different forms: (i) as recombinant peptides fused to pVIII, and (ii) as synthetic peptides conjugated to the phage surface. One peptide (MD10) in its recombinant form produced a stronger anti-peptide antibody response fused to the WT carrier compared to the Delta3 phage carrier, and did not elicit a detectable anti-peptide response in its synthetic form conjugated to either phage carrier. This trend was reversed for a different peptide (4E10(L)), which did not produce a detectable anti-peptide antibody response as a recombinant fusion; yet, as a chemical conjugate to Delta3 phage, but not WT phage, it elicited a highly focused anti-peptide antibody response that exceeded the anti-carrier response by approximately 65-fold. The results suggest that focusing of the antibody response against synthetic peptides can be improved by decreasing the antigenic complexity of the phage surface.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide.Vaccine. 2006 May 8;24(19):4188-200. doi: 10.1016/j.vaccine.2006.01.001. Epub 2006 Jan 11. Vaccine. 2006. PMID: 16488517 Free PMC article.
-
Developing strategies to enhance and focus humoral immune responses using filamentous phage as a model antigen.Bioeng Bugs. 2011 Sep-Oct;2(5):275-83. doi: 10.4161/bbug.2.5.16559. Epub 2011 Sep 1. Bioeng Bugs. 2011. PMID: 22008640 Free PMC article.
-
Homodimeric peptides displayed by the major coat protein of filamentous phage.J Mol Biol. 2000 Jul 7;300(2):307-20. doi: 10.1006/jmbi.2000.3850. J Mol Biol. 2000. PMID: 10873467
-
Filamentous fusion phage cloning vectors for the study of epitopes and design of vaccines.Adv Exp Med Biol. 1989;251:215-8. doi: 10.1007/978-1-4757-2046-4_21. Adv Exp Med Biol. 1989. PMID: 2481962 Review.
-
Immunocontraception: Filamentous Bacteriophage as a Platform for Vaccine Development.Curr Med Chem. 2017 Nov 20;24(35):3907-3920. doi: 10.2174/0929867324666170911160426. Curr Med Chem. 2017. PMID: 28901276 Free PMC article. Review.
Cited by
-
CD4+ T cells provide intermolecular help to generate robust antibody responses in vaccinia virus-vaccinated humans.J Immunol. 2013 Jun 15;190(12):6023-33. doi: 10.4049/jimmunol.1202523. Epub 2013 May 10. J Immunol. 2013. PMID: 23667112 Free PMC article.
-
Bee Venom Immunotherapy: Current Status and Future Directions.Clin Rev Allergy Immunol. 2020 Jun;58(3):326-341. doi: 10.1007/s12016-019-08752-x. Clin Rev Allergy Immunol. 2020. PMID: 31240545 Review.
-
T4 Phage Displaying Dual Antigen Clusters Against H3N2 Influenza Virus Infection.Vaccines (Basel). 2025 Jan 13;13(1):70. doi: 10.3390/vaccines13010070. Vaccines (Basel). 2025. PMID: 39852849 Free PMC article.
-
Phage idiotype vaccination: first phase I/II clinical trial in patients with multiple myeloma.J Transl Med. 2014 May 9;12:119. doi: 10.1186/1479-5876-12-119. J Transl Med. 2014. PMID: 24885819 Free PMC article. Clinical Trial.
-
Beyond phage display: non-traditional applications of the filamentous bacteriophage as a vaccine carrier, therapeutic biologic, and bioconjugation scaffold.Front Microbiol. 2015 Aug 4;6:755. doi: 10.3389/fmicb.2015.00755. eCollection 2015. Front Microbiol. 2015. PMID: 26300850 Free PMC article. Review.
References
-
- Cleveland SM, Buratti E, Jones TD, North P, Baralle F, McLain L, et al. Immunogenic and antigenic dominance of a nonneutralizing epitope over a highly conserved neutralizing epitope in the gp41 envelope glycoprotein of human immunodeficiency virus type 1: its deletion leads to a strong neutralizing response. Virology. 2000;266(1):66–78. - PubMed
-
- Zwick MB. The membrane-proximal external region of HIV-1 gp41: a vaccine target worth exploring. AIDS. 2005;19(16):1725–1737. - PubMed
-
- Inic-Kanada A, Stojanovic M, Zivkovic I, Kosec D, Micic M, Petrusic V, et al. Murine monoclonal antibody 26 raised against tetanus toxoid cross-reacts with beta2-glycoprotein I: its characteristics and role in molecular mimicry. Am J Reprod Immunol. 2009;61(1):39–51. - PubMed
-
- Agarwal A, Sarkar S, Nazabal C, Balasundaram G, Rao KV. B cell responses to a peptide epitope. I. The cellular basis for restricted recognition. J Immunol. 1996;157(7):2779–2788. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

