Evolution of adaptive immune recognition in jawless vertebrates
- PMID: 20056434
- PMCID: PMC2823822
- DOI: 10.1016/j.smim.2009.12.002
Evolution of adaptive immune recognition in jawless vertebrates
Abstract
All extant vertebrates possess an adaptive immune system wherein diverse immune receptors are created and deployed in specialized blood cell lineages. Recent advances in DNA sequencing and developmental resources for basal vertebrates have facilitated numerous comparative analyses that have shed new light on the molecular and cellular bases of immune defense and the mechanisms of immune receptor diversification in the "jawless" vertebrates. With data from these key species in hand, it is becoming possible to infer some general aspects of the early evolution of vertebrate adaptive immunity. All jawed vertebrates assemble their antigen-receptor genes through combinatorial recombination of different "diversity" segments into immunoglobulin or T-cell receptor genes. However, the jawless vertebrates employ an analogous, but independently derived set of immune receptors in order to recognize and bind antigens: the variable lymphocyte receptors (VLRs). The means by which this locus generates receptor diversity and achieves antigen specificity is of considerable interest because these mechanisms represent a completely independent strategy for building a large immune repertoire. Therefore, studies of the VLR system are providing insight into the fundamental principles and evolutionary potential of adaptive immune recognition systems. Here we review and synthesize the wealth of data that have been generated towards understanding the evolution of the adaptive immune system in the jawless vertebrates.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures
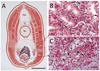
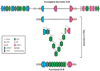
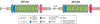
References
-
- Kasahara M, Suzuki T, Pasquier LD. On the origins of the adaptive immune system: novel insights from invertebrates and cold-blooded vertebrates. Trends Immunol. 2004 February;25(2):105–111. - PubMed
-
- Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004 July 8;430(6996):174–180. - PubMed
-
- Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005 December 23;310(5756):1970–1973. - PubMed
-
- Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI, Aravind L, Pancer Z. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007 June;8(6):647–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

