EPR characterization of ascorbyl and sulfur dioxide anion radicals trapped during the reaction of bovine Cytochrome c Oxidase with molecular oxygen
- PMID: 20056464
- PMCID: PMC6446898
- DOI: 10.1016/j.jmr.2009.12.017
EPR characterization of ascorbyl and sulfur dioxide anion radicals trapped during the reaction of bovine Cytochrome c Oxidase with molecular oxygen
Abstract
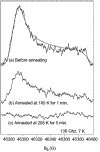
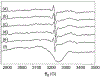
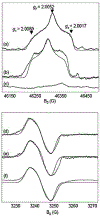
The reaction intermediates of reduced bovine Cytochrome c Oxidase (CcO) were trapped following its reaction with oxygen at 50 micros-6 ms by innovative freeze-quenching methods and studied by EPR. When the enzyme was reduced with either ascorbate or dithionite, distinct radicals were generated; X-band (9 GHz) and D-band (130 GHz) CW-EPR measurements support the assignments of these radicals to ascorbyl and sulfur dioxide anion radical (SO2(-.)), respectively. The X-band spectra show a linewidth of 12 G for the ascorbyl radical and 11 G for the SO2(-.) radical and an isotropic g-value of 2.005 for both species. The D-band spectra reveal clear distinctions in the g-tensors and powder patterns of the two species. The ascorbyl radical spectrum displays approximate axial symmetry with g-values of g(x)=2.0068, g(y)=2.0066, and g(z)=2.0023. The SO2(-.) radical has rhombic symmetry with g-values of g(x)=2.0089, g(y)=2.0052, and g(z)=2.0017. When the contributions from the ascorbyl and SO2(-.) radicals were removed, no protein-based radical on CcO could be identified in the EPR spectra.
2009 Elsevier Inc. All rights reserved.
Figures





References
-
- Buckel W and Golding BT, Radical enzymes in anaerobes, Annu Rev Microbiol 60 (2006) 27–49. - PubMed
-
- Marsh EN, A radical approach to enzyme catalysis, Bioessays 17 (1995) 431–41. - PubMed
-
- Stubbe J and van Der Donk WA, Protein Radicals in Enzyme Catalysis, Chem Rev 98 (1998) 705–762. - PubMed
-
- Sjoberg BM, Reichard P, Graslund A and Ehrenberg A, The tyrosine free radical in ribonucleotide reductase from Escherichia coli, J Biol Chem 253 (1978) 6863–5. - PubMed
-
- Licht S, Gerfen GJ and Stubbe J, Thiyl radicals in ribonucleotide reductases, Science 271 (1996) 477–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

