2,4-Diamino-5-methyl-6-substituted arylthio-furo[2,3-d]pyrimidines as novel classical and nonclassical antifolates as potential dual thymidylate synthase and dihydrofolate reductase inhibitors
- PMID: 20056546
- PMCID: PMC2818873
- DOI: 10.1016/j.bmc.2009.11.029
2,4-Diamino-5-methyl-6-substituted arylthio-furo[2,3-d]pyrimidines as novel classical and nonclassical antifolates as potential dual thymidylate synthase and dihydrofolate reductase inhibitors
Abstract
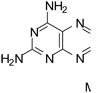
A novel classical antifolate N-{4-[(2,4-diamino-5-methyl-furo[2,3-d]pyrimidin-6-yl)thio]-benzoyl}-l-glutamic acid 5 and 11 nonclassical antifolates 6-16 were designed, synthesized, and evaluated as inhibitors of dihydrofolate reductase (DHFR) and thymidylate synthase (TS). The nonclassical compounds 6-16 were synthesized from 20 via oxidative addition of substituted thiophenols using iodine. Peptide coupling of the intermediate acid 21 followed by saponification gave the classical analog 5. Compound 5 is the first example, to our knowledge, of a 2,4-diamino furo[2,3-d]pyrimidine classical antifolate that has inhibitory activity against both human DHFR and human TS. The classical analog 5 was a nanomolar inhibitor and remarkably selective inhibitor of Pneumocystis carinii DHFR and Mycobacterium avium DHFR at 263-fold and 2107-fold, respectively, compared to mammalian DHFR. The nonclassical analogs 6-16 were moderately potent against pathogen DHFR or TS. This study shows that the furo[2,3-d]pyrimidine scaffold is conducive to dual human DHFR-TS inhibitory activity and to high potency and selectivity for pathogen DHFR.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Design, synthesis, and X-ray crystal structure of a potent dual inhibitor of thymidylate synthase and dihydrofolate reductase as an antitumor agent.J Med Chem. 2000 Oct 19;43(21):3837-51. doi: 10.1021/jm000200l. J Med Chem. 2000. PMID: 11052789
-
Synthesis of N-{4-[(2,4-diamino-5-methyl-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)thio]benzoyl}-L-glutamic acid and N-{4-[(2-amino-4-oxo-5-methyl-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)thio]benzoyl}-L-glutamic acid as dual inhibitors of dihydrofolate reductase and thymidylate synthase and as potential antitumor agents.J Med Chem. 2005 Nov 17;48(23):7215-22. doi: 10.1021/jm058234m. J Med Chem. 2005. PMID: 16279780
-
Synthesis and biological activities of tricyclic conformationally restricted tetrahydropyrido annulated furo[2,3-d]pyrimidines as inhibitors of dihydrofolate reductases.J Med Chem. 1998 Apr 23;41(9):1409-16. doi: 10.1021/jm9705420. J Med Chem. 1998. PMID: 9554874
-
Anticancer antifolates: current status and future directions.Curr Pharm Des. 2003;9(31):2593-613. doi: 10.2174/1381612033453712. Curr Pharm Des. 2003. PMID: 14529544 Review.
-
Antifolate agents: a patent review (2006 - 2010).Expert Opin Ther Pat. 2011 Sep;21(9):1293-308. doi: 10.1517/13543776.2011.587804. Epub 2011 May 27. Expert Opin Ther Pat. 2011. PMID: 21619471 Free PMC article. Review.
Cited by
-
Molecular docking to Toxoplasma gondii thymidylate synthase-dihydrofolate reductase and efficacy of raltitrexed in infected mice.Parasitol Res. 2018 May;117(5):1465-1471. doi: 10.1007/s00436-018-5835-5. Epub 2018 Mar 17. Parasitol Res. 2018. PMID: 29550996
-
Fragment-based discovery of novel thymidylate synthase leads by NMR screening and group epitope mapping.Chem Biol Drug Des. 2010 Sep 1;76(3):218-33. doi: 10.1111/j.1747-0285.2010.01010.x. Epub 2010 Jul 5. Chem Biol Drug Des. 2010. PMID: 20626411 Free PMC article.
-
An innovative strategy for dual inhibitor design and its application in dual inhibition of human thymidylate synthase and dihydrofolate reductase enzymes.PLoS One. 2013;8(4):e60470. doi: 10.1371/journal.pone.0060470. Epub 2013 Apr 5. PLoS One. 2013. PMID: 23577115 Free PMC article.
-
Structural Insights into Mycobacterium tuberculosis Rv2671 Protein as a Dihydrofolate Reductase Functional Analogue Contributing to para-Aminosalicylic Acid Resistance.Biochemistry. 2016 Feb 23;55(7):1107-19. doi: 10.1021/acs.biochem.5b00993. Epub 2016 Feb 5. Biochemistry. 2016. PMID: 26848874 Free PMC article.
-
Crystallographic analysis reveals a novel second binding site for trimethoprim in active site double mutants of human dihydrofolate reductase.J Struct Biol. 2011 Oct;176(1):52-9. doi: 10.1016/j.jsb.2011.06.001. Epub 2011 Jun 13. J Struct Biol. 2011. PMID: 21684339 Free PMC article.
References
-
-
(a) Taken in part from the dissertation submitted by H.J. to the Graduate School of Pharmaceutical Sciences, Duquesne University, in partial fulfillment of the requirements for the degree of Doctor of Philosophy, July 2006. (b) Presented in part at the 228th American Chemical Society National Meeting, Philadelphia, PA, United States, August 22–26, 2004; Abstr. MEDI-101.
-
-
- Schnell JR, Dyson HJ, Wright PE. Annu. Rev. Biophys. Biomol. Struct. 2004;33:119–140. - PubMed
-
- Berman EM, Werbel LM. J. Med. Chem. 1991;34:479–485. - PubMed
-
- Jackman AL, Taylor GA, Gibson W, Kimbell R, Brown M, Calvert AH, Judson IR, Hughes LR. Cancer Res. 1991;51:5579–5586. - PubMed
-
- Taylor EC, Kuhnt D, Shih C, Rinzel SM, Grindey GB, Barredo J, Jannatipour M, Moran R. J. Med. Chem. 1992;35:4450–4454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information

