Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype
- PMID: 20056578
- PMCID: PMC2831952
- DOI: 10.1289/ehp.0901059
Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype
Abstract
Background: Inorganic arsenic is a ubiquitous environmental carcinogen affecting millions of people worldwide. Evolving theory predicts that normal stem cells (NSCs) are transformed into cancer stem cells (CSCs) that then drive oncogenesis. In humans, arsenic is carcinogenic in the urogenital system (UGS), including the bladder and potentially the prostate, whereas in mice arsenic induces multi-organ UGS cancers, indicating that UGS NSCs may represent targets for carcino-genic initiation. However, proof of emergence of CSCs induced by arsenic in a stem cell population is not available.
Methods: We continuously exposed the human prostate epithelial stem/progenitor cell line WPE-stem to an environmentally relevant level of arsenic (5 microM) in vitro and determined the acquired cancer phenotype.
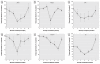
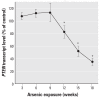
Results: WPE-stem cells rapidly acquired a malignant CSC-like phenotype by 18 weeks of exposure, becoming highly invasive, losing contact inhibition, and hyper-secreting matrix metalloproteinase-9. When hetero-transplanted, these cells (designated As-CSC) formed highly pleomorphic, aggressive tumors with immature epithelial- and mesenchymal-like cells, suggesting a highly pluripotent cell of origin. Consistent with tumor-derived CSCs, As-CSCs formed abundant free-floating spheres enriched in CSC-like cells, as confirmed by molecular analysis and the fact that only these floating cells formed xeno-graft tumors. An early loss of NSC self-renewal gene expression (p63, ABCG2, BMI-1, SHH, OCT-4, NOTCH-1) during arsenite exposure was sub-sequently reversed as the tumor suppressor gene PTEN was progressively suppressed and the CSC-like phenotype acquired.
Conclusions: Arsenite transforms prostate epithelial stem/progenitor cells into CSC-like cells, indicating that it can produce CSCs from a model NSC population.
Figures
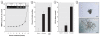




Similar articles
-
Silencing KRAS overexpression in arsenic-transformed prostate epithelial and stem cells partially mitigates malignant phenotype.Toxicol Sci. 2014 Dec;142(2):489-96. doi: 10.1093/toxsci/kfu201. Epub 2014 Sep 30. Toxicol Sci. 2014. PMID: 25273566 Free PMC article.
-
Arsenic-transformed malignant prostate epithelia can convert noncontiguous normal stem cells into an oncogenic phenotype.Environ Health Perspect. 2012 Jun;120(6):865-71. doi: 10.1289/ehp.1204987. Epub 2012 Apr 4. Environ Health Perspect. 2012. PMID: 22472196 Free PMC article.
-
Arsenic-specific stem cell selection during malignant transformation.J Natl Cancer Inst. 2010 May 5;102(9):638-49. doi: 10.1093/jnci/djq093. Epub 2010 Mar 25. J Natl Cancer Inst. 2010. PMID: 20339138 Free PMC article.
-
Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: A novel mechanism of metal carcinogenesis.Semin Cancer Biol. 2019 Aug;57:95-104. doi: 10.1016/j.semcancer.2019.01.002. Epub 2019 Jan 11. Semin Cancer Biol. 2019. PMID: 30641125 Free PMC article. Review.
-
Cancer stem cells and human malignant melanoma.Pigment Cell Melanoma Res. 2008 Feb;21(1):39-55. doi: 10.1111/j.1755-148X.2007.00427.x. Pigment Cell Melanoma Res. 2008. PMID: 18353142 Free PMC article. Review.
Cited by
-
P38/NF-κB/snail pathway is involved in caffeic acid-induced inhibition of cancer stem cells-like properties and migratory capacity in malignant human keratinocyte.PLoS One. 2013;8(3):e58915. doi: 10.1371/journal.pone.0058915. Epub 2013 Mar 13. PLoS One. 2013. PMID: 23516577 Free PMC article.
-
The impact of low-dose carcinogens and environmental disruptors on tissue invasion and metastasis.Carcinogenesis. 2015 Jun;36 Suppl 1(Suppl 1):S128-59. doi: 10.1093/carcin/bgv034. Carcinogenesis. 2015. PMID: 26106135 Free PMC article. Review.
-
LncRNA SNHG11 promotes the malignant transformation of human bronchial epithelial cells induced by beryllium sulfate.Toxicol Res (Camb). 2022 Jun 21;11(4):605-615. doi: 10.1093/toxres/tfac036. eCollection 2022 Aug. Toxicol Res (Camb). 2022. PMID: 36051663 Free PMC article.
-
Early life inorganic lead exposure induces testicular teratoma and renal and urinary bladder preneoplasia in adult metallothionein-knockout mice but not in wild type mice.Toxicology. 2010 Sep 30;276(1):5-10. doi: 10.1016/j.tox.2010.06.006. Epub 2010 Jun 23. Toxicology. 2010. PMID: 20600549 Free PMC article.
-
Nickel-induced down-regulation of ΔNp63 and its role in the proliferation of keratinocytes.Toxicol Appl Pharmacol. 2011 Jun 15;253(3):235-43. doi: 10.1016/j.taap.2011.03.024. Epub 2011 Apr 3. Toxicol Appl Pharmacol. 2011. PMID: 21466819 Free PMC article.
References
-
- Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite induced malignant transformation of human prostate epithelial cells. J Natl Cancer Inst. 2002;94:1888–1891. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Bapat SA. Evolution of cancer stem cells. Sem Cancer Biol. 2007;17:204–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

