Apical surface expression of aspartic protease Plasmepsin 4, a potential transmission-blocking target of the plasmodium ookinete
- PMID: 20056606
- PMCID: PMC2832958
- DOI: 10.1074/jbc.M109.063388
Apical surface expression of aspartic protease Plasmepsin 4, a potential transmission-blocking target of the plasmodium ookinete
Abstract
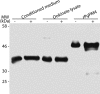
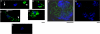
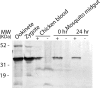
To invade its definitive host, the mosquito, the malaria parasite must cross the midgut peritrophic matrix that is composed of chitin cross-linked by chitin-binding proteins and then develop into an oocyst on the midgut basal lamina. Previous evidence indicates that Plasmodium ookinete-secreted chitinase is important in midgut invasion. The mechanistic role of other ookinete-secreted enzymes in midgut invasion has not been previously examined. De novo mass spectrometry sequencing of a protein obtained by benzamidine affinity column of Plasmodium gallinaceum ookinete axenic culture supernatant demonstrated the presence of an ookinete-secreted plasmepsin, an aspartic protease previously only known to be present in the digestive vacuole of asexual stage malaria parasites. This plasmepsin, the ortholog of Plasmodium falciparum plasmepsin 4, was designated PgPM4. PgPM4 and PgCHT2 (the P. gallinaceum ortholog of P. falciparum chitinase PfCHT1) are both localized on the ookinete apical surface, and both are present in micronemes. Aspartic protease inhibitors (peptidomimetic and natural product), calpain inhibitors, and anti-PgPM4 monoclonal antibodies significantly reduced parasite infectivity for mosquitoes. These results suggest that plasmepsin 4, previously known only to function in the digestive vacuole of asexual blood stage Plasmodium, plays a role in how the ookinete interacts with the mosquito midgut interactions as it becomes an oocyst. These data are the first to delineate a role for an aspartic protease in mediating Plasmodium invasion of the mosquito and demonstrate the potential for plasmepsin 4 as a malaria transmission-blocking vaccine target.
Figures



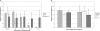
Similar articles
-
Plasmodium falciparum ookinete expression of plasmepsin VII and plasmepsin X.Malar J. 2016 Feb 24;15:111. doi: 10.1186/s12936-016-1161-5. Malar J. 2016. PMID: 26911483 Free PMC article.
-
A Hetero-Multimeric Chitinase-Containing Plasmodium falciparum and Plasmodium gallinaceum Ookinete-Secreted Protein Complex Involved in Mosquito Midgut Invasion.Front Cell Infect Microbiol. 2021 Jan 8;10:615343. doi: 10.3389/fcimb.2020.615343. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33489941 Free PMC article.
-
Identification of novel Plasmodium gallinaceum zygote- and ookinete-expressed proteins as targets for blocking malaria transmission.Infect Immun. 2002 Jan;70(1):102-6. doi: 10.1128/IAI.70.1.102-106.2002. Infect Immun. 2002. PMID: 11748169 Free PMC article.
-
Do malaria ookinete surface proteins P25 and P28 mediate parasite entry into mosquito midgut epithelial cells?Malar J. 2005 Feb 25;4:15. doi: 10.1186/1475-2875-4-15. Malar J. 2005. PMID: 15733320 Free PMC article. Review.
-
Plasmodium ookinete invasion of the mosquito midgut.Curr Top Microbiol Immunol. 2005;295:357-82. doi: 10.1007/3-540-29088-5_14. Curr Top Microbiol Immunol. 2005. PMID: 16265898 Review.
Cited by
-
New ultrastructural analysis of the invasive apparatus of the Plasmodium ookinete.Am J Trop Med Hyg. 2012 Sep;87(3):412-7. doi: 10.4269/ajtmh.2012.11-0609. Epub 2012 Jul 16. Am J Trop Med Hyg. 2012. PMID: 22802443 Free PMC article.
-
Single-Cell Analysis Reveals Distinct Gene Expression and Heterogeneity in Male and Female Plasmodium falciparum Gametocytes.mSphere. 2018 Apr 11;3(2):e00130-18. doi: 10.1128/mSphere.00130-18. Print 2018 Apr 25. mSphere. 2018. PMID: 29643077 Free PMC article.
-
Transmission-Blocking Strategies Against Malaria Parasites During Their Mosquito Stages.Front Cell Infect Microbiol. 2022 Feb 16;12:820650. doi: 10.3389/fcimb.2022.820650. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35252033 Free PMC article. Review.
-
Plasmodium falciparum ookinete expression of plasmepsin VII and plasmepsin X.Malar J. 2016 Feb 24;15:111. doi: 10.1186/s12936-016-1161-5. Malar J. 2016. PMID: 26911483 Free PMC article.
-
A Hetero-Multimeric Chitinase-Containing Plasmodium falciparum and Plasmodium gallinaceum Ookinete-Secreted Protein Complex Involved in Mosquito Midgut Invasion.Front Cell Infect Microbiol. 2021 Jan 8;10:615343. doi: 10.3389/fcimb.2020.615343. eCollection 2020. Front Cell Infect Microbiol. 2021. PMID: 33489941 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

