Non-visual arrestins are constitutively associated with the centrosome and regulate centrosome function
- PMID: 20056609
- PMCID: PMC2832982
- DOI: 10.1074/jbc.M109.062521
Non-visual arrestins are constitutively associated with the centrosome and regulate centrosome function
Abstract
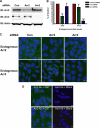
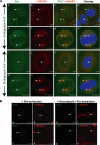
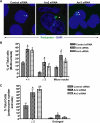
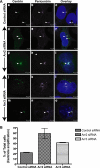
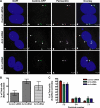
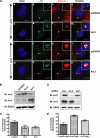
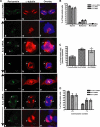
In addition to regulating receptor activity, non-visual arrestins function as scaffolds for numerous intracellular signaling cascades and as regulators of gene transcription. Here we report that the two non-visual arrestins, arrestin2 and arrestin3, localize to the centrosome, a key organelle involved in microtubule nucleation and bipolar mitotic spindle assembly. Both arrestins co-localized with the centrosomal marker gamma-tubulin during interphase and mitosis and were found in purified centrosome preparations. In vitro binding assays demonstrated that both arrestins directly interact with gamma-tubulin. Knockdown of either arrestin by RNA interference resulted in multinucleation, centrosome amplification, and mitotic defects, although only the loss of arrestin2 triggered aberrant microtubule nucleation. Importantly, overexpression of wild type arrestin rescued the multinucleation phenotype and restored normal centrosome number in arrestin siRNA-transfected cells. Moreover, overexpression of arrestin2 or -3 rescued the multinucleation defect observed in MDA-MB-231 breast cancer cells. Taken together, our data reveal that non-visual arrestins are novel centrosomal components and regulate normal centrosome function.
Figures


Similar articles
-
GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation.Nat Cell Biol. 2006 Feb;8(2):137-47. doi: 10.1038/ncb1349. Epub 2005 Dec 25. Nat Cell Biol. 2006. PMID: 16378099
-
Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry.Mol Biol Cell. 2004 Aug;15(8):3642-57. doi: 10.1091/mbc.e03-11-0796. Epub 2004 May 14. Mol Biol Cell. 2004. PMID: 15146056 Free PMC article.
-
beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor.J Biol Chem. 2006 Jan 13;281(2):1261-73. doi: 10.1074/jbc.M506576200. Epub 2005 Nov 9. J Biol Chem. 2006. PMID: 16280323
-
Beta-arrestin signaling and regulation of transcription.J Cell Sci. 2007 Jan 15;120(Pt 2):213-8. doi: 10.1242/jcs.03338. J Cell Sci. 2007. PMID: 17215450 Review.
-
Green fluorescent protein-tagged beta-arrestin translocation as a measure of G protein-coupled receptor activation.Methods Mol Biol. 2004;237:121-6. doi: 10.1385/1-59259-430-1:121. Methods Mol Biol. 2004. PMID: 14501044 Review.
Cited by
-
Differential expression of arrestins is a predictor of breast cancer progression and survival.Breast Cancer Res Treat. 2011 Dec;130(3):791-807. doi: 10.1007/s10549-011-1374-9. Epub 2011 Feb 12. Breast Cancer Res Treat. 2011. PMID: 21318602 Free PMC article.
-
Arrestins regulate cell spreading and motility via focal adhesion dynamics.Mol Biol Cell. 2015 Feb 15;26(4):622-35. doi: 10.1091/mbc.E14-02-0740. Epub 2014 Dec 24. Mol Biol Cell. 2015. PMID: 25540425 Free PMC article.
-
Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons.PLoS One. 2012;7(1):e29239. doi: 10.1371/journal.pone.0029239. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235275 Free PMC article.
-
G Protein-coupled receptor kinase 5 is localized to centrosomes and regulates cell cycle progression.J Biol Chem. 2012 Feb 24;287(9):6928-40. doi: 10.1074/jbc.M111.298034. Epub 2012 Jan 5. J Biol Chem. 2012. PMID: 22223642 Free PMC article.
-
Endocytosis and signaling: cell logistics shape the eukaryotic cell plan.Physiol Rev. 2012 Jan;92(1):273-366. doi: 10.1152/physrev.00005.2011. Physiol Rev. 2012. PMID: 22298658 Free PMC article. Review.
References
-
- Kellogg D. R., Moritz M., Alberts B. M. (1994) Annu. Rev. Biochem. 63, 639–674 - PubMed
-
- Bornens M. (2002) Curr. Opin. Cell Biol. 14, 25–34 - PubMed
-
- King R. W., Peters J. M., Tugendreich S., Rolfe M., Hieter P., Kirschner M. W. (1995) Cell 81, 279–288 - PubMed
-
- Tugendreich S., Tomkiel J., Earnshaw W., Hieter P. (1995) Cell 81, 261–268 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

