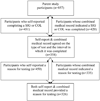
Accuracy of self-reported reason for colorectal cancer testing
- PMID: 20056638
- PMCID: PMC2805466
- DOI: 10.1158/1055-9965.EPI-09-0335
Accuracy of self-reported reason for colorectal cancer testing
Abstract
Assessment of accuracy of self-reported reason for colorectal cancer testing has been limited. We examined the accuracy and correlates of self-reported reason (screening or diagnosis) for having a sigmoidoscopy or colonoscopy. Patients who had received at least one sigmoidoscopy or colonoscopy within the past 5 years were recruited from a large multispecialty clinic in Houston, TX, between 2005 and 2007. We calculated concordance, positive predictive value, negative predictive value, sensitivity, and specificity between self-reported reason and the medical record (gold standard). Logistic regression was performed to identify correlates of accurate self-report. Self-reported reason for testing was more accurate when the sigmoidoscopy or colonoscopy was done for screening, rather than diagnosis. In the multivariable analysis for sigmoidoscopy, age was positively associated with accurately reporting reason for testing, whereas having two or more colorectal cancer tests during the study period (compared with only one test) was negatively associated with accuracy. In the multivariable analysis, none of the correlates was statistically associated with colonoscopy although a similar pattern was observed for number of tests. Determining the best way to identify those who have been tested for diagnosis, rather than screening, is an important next step.
Figures
Similar articles
-
Validation of self-reported colorectal cancer screening behavior from a mixed-mode survey of veterans.Cancer Epidemiol Biomarkers Prev. 2008 Apr;17(4):768-76. doi: 10.1158/1055-9965.EPI-07-0759. Epub 2008 Apr 1. Cancer Epidemiol Biomarkers Prev. 2008. PMID: 18381474
-
Comparison of endoscopic procedures for colorectal cancer screening in women with mammography and Pap smear.Gastrointest Endosc. 2004 Sep;60(3):400-7. doi: 10.1016/s0016-5107(04)01708-0. Gastrointest Endosc. 2004. PMID: 15332031
-
Agreement between self-reported and registered colorectal cancer screening: a meta-analysis.Eur J Cancer Care (Engl). 2015 May;24(3):286-98. doi: 10.1111/ecc.12204. Epub 2014 Apr 23. Eur J Cancer Care (Engl). 2015. PMID: 24754544 Review.
-
Validation of self-reported history of colorectal cancer screening.Can Fam Physician. 2007 Jul;53(7):1192-7. Can Fam Physician. 2007. PMID: 17872816 Free PMC article.
-
Options for screening for colorectal cancer.Scand J Gastroenterol Suppl. 2003;(237):13-6. doi: 10.1080/00855910310001421. Scand J Gastroenterol Suppl. 2003. PMID: 12797674 Review.
Cited by
-
Why not? Understanding the spatial clustering of private facility-based delivery and financial reasons for homebirths in Nigeria.BMC Health Serv Res. 2018 Jun 1;18(1):397. doi: 10.1186/s12913-018-3225-4. BMC Health Serv Res. 2018. PMID: 29859092 Free PMC article.
-
Racial and ethnic variations in the effects of family history of colorectal cancer on screening compliance.Gastroenterology. 2013 Oct;145(4):775-81.e2. doi: 10.1053/j.gastro.2013.06.037. Epub 2013 Jun 22. Gastroenterology. 2013. PMID: 23796457 Free PMC article.
-
Communication of genetic test results to family and health-care providers following disclosure of research results.Genet Med. 2014 Apr;16(4):294-301. doi: 10.1038/gim.2013.137. Epub 2013 Oct 3. Genet Med. 2014. PMID: 24091800 Free PMC article.
-
Engaging Patients in Decisions About Cancer Screening: Exploring the Decision Journey Through the Use of a Patient Portal.Am J Prev Med. 2018 Feb;54(2):237-247. doi: 10.1016/j.amepre.2017.10.027. Epub 2017 Dec 11. Am J Prev Med. 2018. PMID: 29241715 Free PMC article.
-
The prognostic impact of BMI on colorectal cancer is stratified by tumor location.Front Oncol. 2022 Nov 7;12:987518. doi: 10.3389/fonc.2022.987518. eCollection 2022. Front Oncol. 2022. PMID: 36419882 Free PMC article.
References
-
- Shen J, Samson L, Washington E, Johnson P, Edwards C, Malone A. Barriers of HIPAA regulation to implementation of health services research. J Med Syst. 2006;30:65–69. - PubMed
-
- Partin MR, Grill J, Noorbaloochi S, et al. Validation of self-reported colorectal cancer screening behavior from a mixed-mode survey of veterans. Cancer Epidemiol Biomarkers Prev. 2008;17:768–776. - PubMed
-
- Vernon SW, Tiro JA, Vojvodic RW, et al. Reliability and validity of a questionnaire to measure colorectal cancer screening behaviors: does mode of survey administration matter? Cancer Epidemiol Biomarkers Prev. 2008;17:758–767. - PubMed
-
- Vernon SW, Meissner H, Klabunde C, Rimer BK, Ahnen DJ, Bastani R, et al. Measures for ascertaining use of colorectal cancer screening in behavioral, health services, and epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2004;13(6):898–905. - PubMed
-
- Last JM. A dictionary for public health. New York: Oxford University Press; 2007. p. 336.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous