Possible molecular mechanisms underlying age-related cardiomyocyte apoptosis in the F344XBN rat heart
- PMID: 20056683
- PMCID: PMC2806239
- DOI: 10.1093/gerona/glp203
Possible molecular mechanisms underlying age-related cardiomyocyte apoptosis in the F344XBN rat heart
Abstract
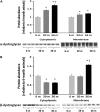
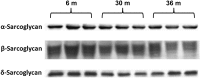
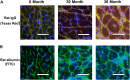
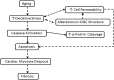
Despite advances in treatment, age-related cardiac dysfunction still remains a leading cause of cardiovascular death. Recent data have suggested that increases in cardiomyocyte apoptosis may be involved in the pathological remodeling of heart. Here, we examine the effects of aging on cardiomyocyte apoptosis in 6-, 30-, and 36-month-old Fischer344 x Brown Norway F1 hybrid rats (F344XBN). Compared with 6-month hearts, aged hearts exhibited increased TdT-mediated dUTP nick end labeling-positive nuclei, caspase-3 activation, caspase-dependent cleavage of alpha-fodrin and diminished phosphorylation of protein kinase B/Akt (Thr 308). These age-dependent increases in cardiomyocyte apoptosis were associated with alterations in the composition of the cardiac dystrophin glycoprotein complex and elevated cytoplasmic IgG and albumin immunoreactivity. Immunohistochemical analysis confirmed these data and demonstrated qualitative differences in localization of dystrophin-glycoprotein complex (DGC) molecules with aging. Taken together, these data suggest that aging-related increases in cardiac apoptotic activity model may be due, at least in part, to age-associated changes in DGC structure.
Figures

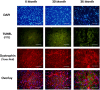
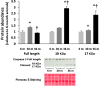
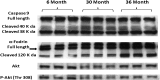
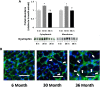
References
-
- Lakatta EG. Aging and cardiovascular structure and function in healthy sedentary humans. Aging (Milano) 1998;10:162–164. - PubMed
-
- Hacker TA, McKiernan SH, Douglas PS, Wanagat J, Aiken JM. Age-related changes in cardiac structure and function in Fischer 344 x Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol. 2006;290:H304–H311. - PubMed
-
- Walker EM, Jr, Nillas MS, Mangiarua EI, et al. Age-associated changes in hearts of male Fischer 344/Brown Norway F1 rats. Ann Clin Lab Sci. 2006;36:427–438. - PubMed
-
- Asano S, Rice KM, Kakarla S, et al. Aging influences multiple indices of oxidative stress in the heart of the Fischer 344/NNia x Brown Norway/BiNia rat. Redox Rep. 2007;12:167–180. - PubMed
-
- Tsutsui H, Ide T, Kinugawa S. Mitochondrial oxidative stress, DNA damage, and heart failure. Antioxid Redox Signal. 2006;8:1737–1744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

