Angiotensin II promotes development of the renal microcirculation through AT1 receptors
- PMID: 20056745
- PMCID: PMC2831863
- DOI: 10.1681/ASN.2009010045
Angiotensin II promotes development of the renal microcirculation through AT1 receptors
Abstract
Pharmacologic or genetic deletion of components of the renin-angiotensin system leads to postnatal kidney injury, but the roles of these components in kidney development are unknown. To test the hypothesis that angiotensin II supports angiogenesis during postnatal kidney development, we quantified CD31(+) postglomerular microvessels, performed quantitative PCR analysis of vascular growth factor expression, and measured renal blood flow by magnetic resonance. Treating rats with the angiotensin II type 1 receptor antagonist candesartan for 2 weeks after birth reduced the total length, volume, and surface area of capillaries in both the cortex and the medulla and inhibited the organization of vasa recta bundles. In addition, angiotensin II type 1 antagonism inhibited the transcription of angiogenic growth factors vascular endothelial growth factor, angiopoietin-1, angiopoietin-2, and the angiopoietin receptor Tie-2 in cortex and medulla. Similarly, Agtr1a(-/-);Agtr1b(-/-) mouse kidneys had decreased angiopoietin-1, angiopoietin-2, and Tie-2 mRNAs at postnatal day 14. To test whether increased urinary flow leads to microvascular injury, we induced postnatal polyuria with either lithium or adrenalectomy, but these did not alter vascular endothelial growth factor expression or vasa recta organization. Compared with vehicle-treated rats, renal blood flow was significantly (approximately 20%) lower in candesartan-treated rats even 14 days after candesartan withdrawal. Taken together, these data demonstrate that angiotensin II promotes postnatal expansion of postglomerular capillaries and organization of vasa recta bundles, which are necessary for development of normal renal blood flow.
Figures
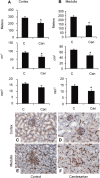

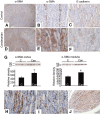

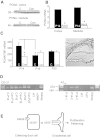
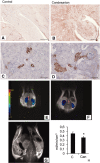
Comment in
-
Dependence of renal microvessel density on angiotensin II: only in the fetus?J Am Soc Nephrol. 2010 Mar;21(3):386-8. doi: 10.1681/ASN.2010010069. Epub 2010 Feb 18. J Am Soc Nephrol. 2010. PMID: 20167704 Free PMC article. No abstract available.
Similar articles
-
Temporal renal expression of angiogenic growth factors and their receptors in experimental diabetes: role of the renin-angiotensin system.J Hypertens. 2005 Jan;23(1):153-64. doi: 10.1097/00004872-200501000-00026. J Hypertens. 2005. PMID: 15643138
-
Vascular endothelial growth factor signaling is necessary for expansion of medullary microvessels during postnatal kidney development.Am J Physiol Renal Physiol. 2016 Sep 1;311(3):F586-99. doi: 10.1152/ajprenal.00221.2016. Epub 2016 Jul 13. Am J Physiol Renal Physiol. 2016. PMID: 27413199
-
Regulation of endothelial proliferation by the renin-angiotensin system in human umbilical vein endothelial cells.Reproduction. 2008 Jul;136(1):125-30. doi: 10.1530/REP-07-0374. Epub 2008 Apr 4. Reproduction. 2008. PMID: 18390690
-
Angiopoietin growth factors and Tie receptor tyrosine kinases in renal vascular development.Pediatr Nephrol. 2001 Feb;16(2):177-84. doi: 10.1007/s004670000509. Pediatr Nephrol. 2001. PMID: 11261688 Review.
-
Renal responses to AT1 receptor blockade.Am J Hypertens. 2000 Jan;13(1 Pt 2):45S-54S. doi: 10.1016/s0895-7061(99)00248-4. Am J Hypertens. 2000. PMID: 10678288 Review.
Cited by
-
Calcineurin-inhibition Results in Upregulation of Local Renin and Subsequent Vascular Endothelial Growth Factor Production in Renal Collecting Ducts.Transplantation. 2016 Feb;100(2):325-333. doi: 10.1097/TP.0000000000000961. Transplantation. 2016. PMID: 26502369 Free PMC article.
-
Renin-angiotensin system in ureteric bud branching morphogenesis: implications for kidney disease.Pediatr Nephrol. 2014 Apr;29(4):609-20. doi: 10.1007/s00467-013-2616-3. Epub 2013 Sep 7. Pediatr Nephrol. 2014. PMID: 24061643 Review.
-
Endothelial Injury Syndromes after Allogeneic Hematopoietic Stem Cell Transplantation: Angiopetin-2 as a Novel Predictor of the Outcome and the Role of Functional Autoantibodies against Angiotensin II Type 1 and Endothelin A Receptor.Int J Mol Sci. 2023 Apr 9;24(8):6960. doi: 10.3390/ijms24086960. Int J Mol Sci. 2023. PMID: 37108124 Free PMC article.
-
When Less or More Isn't Enough: Renal Maldevelopment Arising From Disequilibrium in the Renin-Angiotensin System.Front Pediatr. 2019 Jul 17;7:296. doi: 10.3389/fped.2019.00296. eCollection 2019. Front Pediatr. 2019. PMID: 31380328 Free PMC article. Review.
-
Antibodies against Angiotensin II Type 1 and Endothelin A Receptors: Relevance and pathogenicity.Hum Immunol. 2019 Aug;80(8):561-567. doi: 10.1016/j.humimm.2019.04.012. Epub 2019 Apr 19. Hum Immunol. 2019. PMID: 31010696 Free PMC article. Review.
References
-
- Cha J-H, Kim Y-H, Jung J-Y, Han K-H, Madsen KM, Kim J: Cell proliferation in the loop of Henle in the developing rat kidney. J Am Soc Nephrol 12: 1410–1421, 2001 - PubMed
-
- Friberg P, Sundelin B, Bohman S-O, Bobik A, Nilsson H, Wickman A, Gustafsson H, Petersen J, Adams MA: Renin-angiotensin system in neonatal rats: Induction of a renal abnormality in response to ACE inhibition or angiotensin II antagonism. Kidney Int 45: 485–492, 1994 - PubMed
-
- Guron G, Adams MA, Sundelin B, Friberg P: Neonatal angiotensin-converting enzyme inhibition in the rat induces persistent abnormalities in renal function and histology. Hypertension 29: 91–97, 1997 - PubMed
-
- Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA: Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension 29: 216–221, 1997 - PubMed
-
- Nagata M, Tanimoto K, Fukamizu A, Kon Y, Sugiyama F, Yagami K, Murakami K, Watanabe T: Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest 75: 745–753, 1996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

