Angiotensin II promotes development of the renal microcirculation through AT1 receptors
- PMID: 20056745
- PMCID: PMC2831863
- DOI: 10.1681/ASN.2009010045
Angiotensin II promotes development of the renal microcirculation through AT1 receptors
Abstract
Pharmacologic or genetic deletion of components of the renin-angiotensin system leads to postnatal kidney injury, but the roles of these components in kidney development are unknown. To test the hypothesis that angiotensin II supports angiogenesis during postnatal kidney development, we quantified CD31(+) postglomerular microvessels, performed quantitative PCR analysis of vascular growth factor expression, and measured renal blood flow by magnetic resonance. Treating rats with the angiotensin II type 1 receptor antagonist candesartan for 2 weeks after birth reduced the total length, volume, and surface area of capillaries in both the cortex and the medulla and inhibited the organization of vasa recta bundles. In addition, angiotensin II type 1 antagonism inhibited the transcription of angiogenic growth factors vascular endothelial growth factor, angiopoietin-1, angiopoietin-2, and the angiopoietin receptor Tie-2 in cortex and medulla. Similarly, Agtr1a(-/-);Agtr1b(-/-) mouse kidneys had decreased angiopoietin-1, angiopoietin-2, and Tie-2 mRNAs at postnatal day 14. To test whether increased urinary flow leads to microvascular injury, we induced postnatal polyuria with either lithium or adrenalectomy, but these did not alter vascular endothelial growth factor expression or vasa recta organization. Compared with vehicle-treated rats, renal blood flow was significantly (approximately 20%) lower in candesartan-treated rats even 14 days after candesartan withdrawal. Taken together, these data demonstrate that angiotensin II promotes postnatal expansion of postglomerular capillaries and organization of vasa recta bundles, which are necessary for development of normal renal blood flow.
Figures
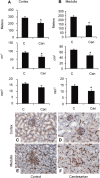

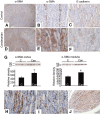

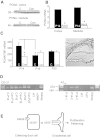
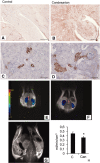
Comment in
-
Dependence of renal microvessel density on angiotensin II: only in the fetus?J Am Soc Nephrol. 2010 Mar;21(3):386-8. doi: 10.1681/ASN.2010010069. Epub 2010 Feb 18. J Am Soc Nephrol. 2010. PMID: 20167704 Free PMC article. No abstract available.
References
-
- Cha J-H, Kim Y-H, Jung J-Y, Han K-H, Madsen KM, Kim J: Cell proliferation in the loop of Henle in the developing rat kidney. J Am Soc Nephrol 12: 1410–1421, 2001 - PubMed
-
- Friberg P, Sundelin B, Bohman S-O, Bobik A, Nilsson H, Wickman A, Gustafsson H, Petersen J, Adams MA: Renin-angiotensin system in neonatal rats: Induction of a renal abnormality in response to ACE inhibition or angiotensin II antagonism. Kidney Int 45: 485–492, 1994 - PubMed
-
- Guron G, Adams MA, Sundelin B, Friberg P: Neonatal angiotensin-converting enzyme inhibition in the rat induces persistent abnormalities in renal function and histology. Hypertension 29: 91–97, 1997 - PubMed
-
- Hilgers KF, Reddi V, Krege JH, Smithies O, Gomez RA: Aberrant renal vascular morphology and renin expression in mutant mice lacking angiotensin-converting enzyme. Hypertension 29: 216–221, 1997 - PubMed
-
- Nagata M, Tanimoto K, Fukamizu A, Kon Y, Sugiyama F, Yagami K, Murakami K, Watanabe T: Nephrogenesis and renovascular development in angiotensinogen-deficient mice. Lab Invest 75: 745–753, 1996 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

