Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy
- PMID: 20056750
- PMCID: PMC2831855
- DOI: 10.1681/ASN.2009060571
Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy
Abstract
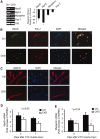
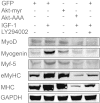
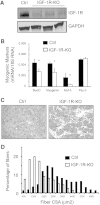
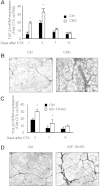
Muscle wasting in chronic kidney disease (CKD) begins with impaired insulin/IGF-1 signaling, causing abnormal protein metabolism. In certain models of muscle atrophy, reduced satellite cell function contributes to atrophy, but how CKD affects satellite cell function is unknown. Here, we found that isolated satellite cells from mice with CKD had less MyoD, the master switch of satellite cell activation, and suppressed myotube formation compared with control mice. In vivo, CKD delayed the regeneration of injured muscle and decreased MyoD and myogenin expression, suggesting that CKD impairs proliferation and differentiation of satellite cells. In isolated satellite cells from control mice, IGF-1 increased the expression of myogenic genes through an Akt-dependent pathway. CKD impaired Akt phosphorylation in satellite cells after muscle injury. To test whether impaired IGF-1 signaling could be responsible for decreased satellite cell function in CKD, we created an inducible IGF-1 receptor knockout mouse and found impaired satellite cell function and muscle regeneration. In addition, both CKD and IGF-1 receptor knockout mice developed fibrosis in regenerating muscles. Taken together, impaired IGF-1 signaling in CKD not only leads to abnormal protein metabolism in muscle but also impairs satellite cell function and promotes fibrosis in regenerating muscle. These signaling pathways may hold potential therapeutic targets to reduce CKD-related muscle wasting.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

