GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity
- PMID: 20056751
- PMCID: PMC2831860
- DOI: 10.1681/ASN.2009060672
GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity
Abstract
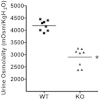
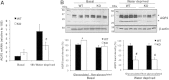
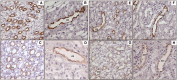
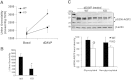
Glycogen synthase kinase 3beta (GSK3beta), a serine/threonine protein kinase, is a key target of drug discovery in several diseases, including diabetes and Alzheimer disease. Because lithium, a potent inhibitor of GSK3beta, causes nephrogenic diabetes insipidus, GSK3beta may play a crucial role in regulating water homeostasis. We developed renal collecting duct-specific GSK3beta knockout mice to determine whether deletion of GSK3beta affects arginine vasopressin-dependent renal water reabsorption. Although only mildly polyuric under normal conditions, knockout mice exhibited an impaired urinary concentrating ability in response to water deprivation or treatment with a vasopressin analogue. The knockout mice had reduced levels of mRNA, protein, and membrane localization of the vasopressin-responsive water channel aquaporin 2 compared with wild-type mice. The knockout mice also expressed lower levels of pS256-AQP2, a phosphorylated form crucial for membrane trafficking. Levels of cAMP, a major regulator of aquaporin 2 expression and trafficking, were also lower in the knockout mice. Both GSK3beta gene deletion and pharmacologic inhibition of GSK3beta reduced adenylate cyclase activity. In summary, GSK3beta inactivation or deletion reduces aquaporin 2 expression by modulating adenylate cyclase activity and cAMP generation, thereby impairing responses to vasopressin in the renal collecting duct.
Figures



References
-
- Mukai F, Ishiguro K, Sano Y, Fujita SC: Alternative splicing isoform of tau protein kinase I/glycogen synthase kinase 3beta. J Neurochem 81: 1073–1083, 2002 - PubMed
-
- MacAulay K, Doble BW, Patel S, Hansotia T, Sinclair EM, Drucker DJ, Nagy A, Woodgett JR: Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab 6: 329–337, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

