A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease
- PMID: 20056760
- PMCID: PMC2827596
- DOI: 10.2215/CJN.07131009
A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease
Abstract
Background and objectives: The optimal treatment of secondary hyperparathyroidism in chronic kidney disease (CKD) is unknown.
Design, setting, participants, & measurements: We conducted a randomized, blinded, 3-month trial in vitamin D-deficient CKD stage 3 and 4 patients with parathyroid hormone (PTH) values above the Kidney Disease Outcomes Quality Initiative target, comparing cholecalciferol (4000 IU/d x 1 month, then 2000 IU/d; n = 22) to doxercalciferol (1 microg/d; n = 25).
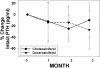
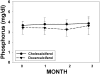
Results: There was no difference in baseline demographics or lab tests, except a slightly higher estimated GFR (eGFR) in the doxercalciferol group. There was a significant increase in vitamin D level in the cholecalciferol group (14 +/- 6 to 37 +/- 10 ng/ml; P < 0.001) but no change in the doxercalciferol group. The PTH decreased by 27% +/- 34% in the doxercalciferol group (P = 0.002) and decreased by 10% +/- 31% in the cholecalciferol group (P = 0.16), but the difference between treatments was NS (P = 0.11). Similar results were found when absolute PTH change from baseline to end point was analyzed in a repeated-measures ANOVA model. The serum calcium and urine calcium excretions were not different. Additional non-mineral-related end points, albuminuria, and BP were evaluated, and although trends were present, this did not reach significance.
Conclusions: This prospective, randomized trial demonstrated a within-group reduction in PTH in the doxercalciferol-treated patients but no significant difference between the doxercalciferol and cholecalciferol patients. Larger, long-term studies are needed to demonstrate efficacy of mineral-related and non-mineral-related end points and safety.
Figures

References
-
- LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM: Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis 45: 1026–1033, 2005 - PubMed
-
- Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR: Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30: 771–777, 2002 - PubMed
-
- Elder GJ, Mackun K: 25-Hydroxyvitamin D deficiency and diabetes predict reduced BMD in patients with chronic kidney disease. J Bone Miner Res 21: 1778–1784, 2006 - PubMed
-
- Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ: Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol, 24: 503–510, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

