Vinclozolin exposure in utero induces postpubertal prostatitis and reduces sperm production via a reversible hormone-regulated mechanism
- PMID: 20056826
- PMCID: PMC2817613
- DOI: 10.1210/en.2009-0982
Vinclozolin exposure in utero induces postpubertal prostatitis and reduces sperm production via a reversible hormone-regulated mechanism
Abstract
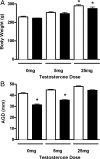
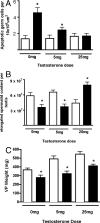
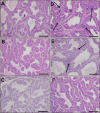
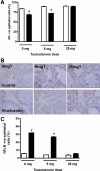
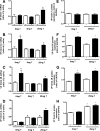
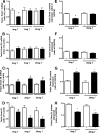
Vinclozolin is an endocrine-disrupting chemical (EDC) that binds with high affinity to the androgen receptor (AR) and blocks the action of gonadal hormones on male reproductive organs. An alternative mechanism of action of Vinclozolin involves transgenerational effects on the male reproductive tract. We previously reported in utero Vinclozolin exposure-induced prostatitis (prostate inflammation) in postpubertal rats concurrent with down-regulation of AR and increased nuclear factor-kappaB activation. We postulated the male reproductive abnormalities induced by in utero Vinclozolin exposure could be reversed by testosterone supplementation, in contrast to the permanent modifications involving DNA methyltransferases (Dnmts) described by others. To test this hypothesis, we administered high-dose testosterone at puberty to Vinclozolin-treated rats and determined the effect on anogenital distance (AGD); testicular germ cell apoptosis, concentration of elongated spermatids, and the onset of prostatitis. Concurrently we examined Dnmt1, -3A, -3B, and -3L mRNA expression. Consistent with previous reports, in utero exposure to Vinclozolin significantly reduced AGD, increased testicular germ cell apoptosis 3-fold, reduced elongated spermatid number by 40%, and induced postpubertal prostatitis in 100% of exposed males. Administration of high-dose testosterone (25 mg/kg) at puberty normalized AGD, reduced germ cell apoptosis, and restored elongated spermatid number. Testosterone restored AR and nuclear factor-kappaB expression in the prostate and abolished Vinclozolin-induced prostatitis. Altered Dnmt expression was evident with in utero Vinclozolin exposure and was not normalized after testosterone treatment. These data demonstrate in utero Vinclozolin-induced male reproductive tract abnormalities are AR mediated and reversible and involve a mechanism independent of Dnmt expression.
Figures
Similar articles
-
Early-onset endocrine disruptor-induced prostatitis in the rat.Environ Health Perspect. 2008 Jul;116(7):923-9. doi: 10.1289/ehp.11239. Environ Health Perspect. 2008. PMID: 18629315 Free PMC article.
-
Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat.Toxicol Ind Health. 1999 Jan-Mar;15(1-2):65-79. doi: 10.1177/074823379901500107. Toxicol Ind Health. 1999. PMID: 10188192
-
Transgenerational effects of the endocrine disruptor vinclozolin on the prostate transcriptome and adult onset disease.Prostate. 2008 Apr 1;68(5):517-29. doi: 10.1002/pros.20724. Prostate. 2008. PMID: 18220299 Free PMC article.
-
Epigenetic transgenerational actions of vinclozolin on the development of disease and cancer.Crit Rev Oncog. 2007 Aug;13(1):75-82. doi: 10.1615/critrevoncog.v13.i1.30. Crit Rev Oncog. 2007. PMID: 17956218 Free PMC article. Review.
-
Anogenital distance as a toxicological or clinical marker for fetal androgen action and risk for reproductive disorders.Arch Toxicol. 2019 Feb;93(2):253-272. doi: 10.1007/s00204-018-2350-5. Epub 2018 Nov 14. Arch Toxicol. 2019. PMID: 30430187 Review.
Cited by
-
The relationship between anogenital distance and the androgen receptor CAG repeat length.Asian J Androl. 2013 Mar;15(2):286-9. doi: 10.1038/aja.2012.126. Epub 2013 Jan 21. Asian J Androl. 2013. PMID: 23334200 Free PMC article.
-
Endocrine Disruptors and Prostate Cancer.Int J Mol Sci. 2022 Jan 21;23(3):1216. doi: 10.3390/ijms23031216. Int J Mol Sci. 2022. PMID: 35163140 Free PMC article. Review.
-
Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):7034-9. doi: 10.1073/pnas.1015935108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482758 Free PMC article.
-
Anogenital distance in the etiology of chronic prostatitis: does it lead to novel surgical treatments?Front Urol. 2025 Jan 7;4:1418007. doi: 10.3389/fruro.2024.1418007. eCollection 2024. Front Urol. 2025. PMID: 40777105 Free PMC article. No abstract available.
-
Androgen signaling disruption during fetal and postnatal development affects androgen receptor and connexin 43 expression and distribution in adult boar prostate.Biomed Res Int. 2013;2013:407678. doi: 10.1155/2013/407678. Epub 2013 Sep 17. Biomed Res Int. 2013. PMID: 24151599 Free PMC article.
References
-
- Colborn T, Dumanoski D, Myers JP 1996 Our stolen future. Are we threatening our fertility, intelligence and survival? A scientific detective story. Boston: Little, Brown and Co.
-
- Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK 2003 Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst 95:1276–1299 - PubMed
-
- Kelce WR, Gray L, Wilson EM 1998 Antiandrogens as environmental endocrine disruptors. Reprod Fertil Dev 10:105–111 - PubMed
-
- Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray Jr LE 1994 Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol 126:276–285 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

