Acute insulin signaling in pancreatic beta-cells is mediated by multiple Raf-1 dependent pathways
- PMID: 20056832
- PMCID: PMC2817610
- DOI: 10.1210/en.2009-0678
Acute insulin signaling in pancreatic beta-cells is mediated by multiple Raf-1 dependent pathways
Abstract
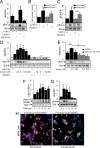
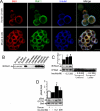
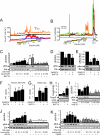
Insulin enhances the proliferation and survival of pancreatic beta-cells, but its mechanisms remain unclear. We hypothesized that Raf-1, a kinase upstream of both ERK and Bad, might be a critical target of insulin in beta-cells. To test this hypothesis, we treated human and mouse islets as well as MIN6 beta-cells with multiple insulin concentrations and examined putative downstream targets using immunoblotting, immunoprecipitation, quantitative fluorescent imaging, and cell death assays. Low doses of insulin rapidly activated Raf-1 by dephosphorylating serine 259 and phosphorylating serine 338 in human islets, mouse islets, and MIN6 cells. The phosphorylation of ERK by insulin was eliminated by exposure to a Raf inhibitor (GW5074) or transfection with a dominant-negative Raf-1 mutant. Insulin also enhanced the interaction between mitochondrial Raf-1 and Bcl-2 agonist of cell death (Bad), promoting Bad inactivation via its phosphorylation on serine 112. Insulin-stimulated ERK phosphorylation was abrogated by calcium chelation, calcineurin and calmodulin-dependent protein kinase II inhibitors, and Ned-19, a nicotinic acid adenine dinucleotide phosphate receptor (NAADPR) antagonist. Blocking Raf-1 and Ca(2+) signaling resulted in nonadditive beta-cell death. Autocrine insulin signaling partly accounted for the effects of glucose on ERK phosphorylation. Our results demonstrate that Raf-1 is a critical target of insulin in primary beta-cells. Activation of Raf-1 leads to both an ERK-dependent pathway that involves nicotinic acid adenine dinucleotide phosphate-sensitive Ca(2+) stores and Ca(2+)-dependent phosphorylation events, and an ERK-independent pathway that involves Bad inactivation at the mitochondria. Together our findings identify a novel insulin signaling pathway in beta-cells and shed light on insulin's antiapoptotic and mitogenic mechanisms.
Figures
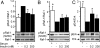

References
-
- Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN 2006 Total insulin and IGF-I resistance in pancreatic β cells causes overt diabetes. Nat Genet 38:583–588 - PubMed
-
- Hashimoto N, Kido Y, Uchida T, Asahara S, Shigeyama Y, Matsuda T, Takeda A, Tsuchihashi D, Nishizawa A, Ogawa W, Fujimoto Y, Okamura H, Arden KC, Herrera PL, Noda T, Kasuga M 2006 Ablation of PDK1 in pancreatic β cells induces diabetes as a result of loss of β cell mass. Nat Genet 38:589–593 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

