Involvement of phosphoinositide 3-kinase gamma in angiogenesis and healing of experimental myocardial infarction in mice
- PMID: 20056919
- PMCID: PMC2833289
- DOI: 10.1161/CIRCRESAHA.109.207449
Involvement of phosphoinositide 3-kinase gamma in angiogenesis and healing of experimental myocardial infarction in mice
Abstract
Rationale: Phosphoinositide 3-kinase (PI3K)gamma is expressed in hematopoietic cells, endothelial cells (ECs), and cardiomyocytes and regulates different cellular functions relevant to inflammation, tissue remodeling and cicatrization. Recently, PI3Kgamma inhibitors have been indicated for the treatment of chronic inflammatory/autoimmune diseases and atherosclerosis.
Objective: We aimed to determine PI3Kgamma contribution to the angiogenic capacity of ECs and the effect of PI3Kgamma inhibition on healing of myocardial infarction (MI).
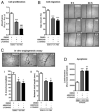
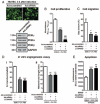
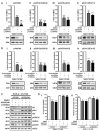
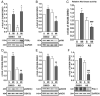
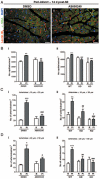
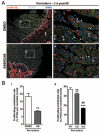
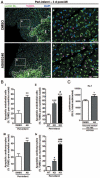
Methods and results: Human umbilical ECs were treated with a selective PI3Kgamma inhibitor, AS605240, or a pan-phosphoinositide 3-kinases inhibitor, LY294002. Both inhibitory treatments and small interfering RNA-mediated PI3Kgamma knockdown strongly impaired ECs angiogenic capacity, because of suppression of the PI3K/Akt and mitogen-activated protein kinase pathways. Constitutive activation of Akt rescued the angiogenic defect. Reparative angiogenesis was studied in vivo in a model of MI. AS605240 did not affect MI-induced PI3Kgamma upregulation, whereas it suppressed Akt activation and downstream signaling. AS605240 strongly reduced inflammation, enhanced cardiomyocyte apoptosis, and impaired survival and proliferation of ECs in peri-infarct zone, which resulted in defective reparative neovascularization. As a consequence, AS605240-treated MI hearts showed increased infarct size and impaired recovery of left ventricular function. Similarly, PI3Kgamma-deficient mice showed impaired reparative neovascularization, enhanced cardiomyocyte apoptosis and marked deterioration of cardiac function following MI. Mice expressing catalytically inactive PI3Kgamma also failed to mount a proper neovascularization, although cardiac dysfunction was similar to wild-type controls.
Conclusions: PI3Kgamma expression and catalytic activity are involved at different levels in reparative neovascularization and healing of MI.
Figures
Comment in
-
Effects of PI3Kgamma inhibition using AS-605240 in acute myocardial infarction.Circ Res. 2010 Jul 23;107(2):e5; author reply e6-7. doi: 10.1161/CIRCRESAHA.110.224568. Circ Res. 2010. PMID: 20651289 No abstract available.
Similar articles
-
Attenuation of cardiac dysfunction and remodeling of myocardial infarction by microRNA-130a are mediated by suppression of PTEN and activation of PI3K dependent signaling.J Mol Cell Cardiol. 2015 Dec;89(Pt A):87-97. doi: 10.1016/j.yjmcc.2015.10.011. Epub 2015 Oct 13. J Mol Cell Cardiol. 2015. PMID: 26458524 Free PMC article.
-
Midkine prevents ventricular remodeling and improves long-term survival after myocardial infarction.Am J Physiol Heart Circ Physiol. 2009 Feb;296(2):H462-9. doi: 10.1152/ajpheart.00733.2008. Epub 2008 Dec 5. Am J Physiol Heart Circ Physiol. 2009. PMID: 19060126
-
Nerve growth factor promotes cardiac repair following myocardial infarction.Circ Res. 2010 Apr 16;106(7):1275-84. doi: 10.1161/CIRCRESAHA.109.210088. Epub 2010 Apr 1. Circ Res. 2010. PMID: 20360245 Free PMC article.
-
Angiogenesis after acute myocardial infarction.Cardiovasc Res. 2021 Apr 23;117(5):1257-1273. doi: 10.1093/cvr/cvaa287. Cardiovasc Res. 2021. PMID: 33063086 Review.
-
PI3K signalling in inflammation.Biochim Biophys Acta. 2015 Jun;1851(6):882-97. doi: 10.1016/j.bbalip.2014.12.006. Epub 2014 Dec 13. Biochim Biophys Acta. 2015. PMID: 25514767 Review.
Cited by
-
Phosphoinositide 3-kinase γ inhibits cardiac GSK-3 independently of Akt.Sci Signal. 2013 Jan 22;6(259):ra4. doi: 10.1126/scisignal.2003308. Sci Signal. 2013. PMID: 23354687 Free PMC article.
-
8e Protects against Acute Cerebral Ischemia by Inhibition of PI3Kγ-Mediated Superoxide Generation in Microglia.Molecules. 2018 Oct 31;23(11):2828. doi: 10.3390/molecules23112828. Molecules. 2018. PMID: 30384445 Free PMC article.
-
Intramyocardial administration of chimeric ephrinA1-Fc promotes tissue salvage following myocardial infarction in mice.J Physiol. 2011 Apr 1;589(Pt 7):1725-40. doi: 10.1113/jphysiol.2010.202366. Epub 2011 Jan 31. J Physiol. 2011. PMID: 21282286 Free PMC article.
-
Effects of Panax quinquefolium saponin on phosphatidylinositol 3-kinase/serine threonine kinase pathway of neonatal rat myocardial cells subjected to hypoxia.Chin J Integr Med. 2015 May;21(5):384-8. doi: 10.1007/s11655-014-1881-8. Epub 2014 Jul 14. Chin J Integr Med. 2015. PMID: 25022552
-
Inhibition of Phosphoinositide 3-Kinase Gamma Protects Endothelial Cells via the Akt Signaling Pathway in Sepsis-Induced Acute Kidney Injury.Kidney Blood Press Res. 2022;47(10):616-630. doi: 10.1159/000526916. Epub 2022 Sep 21. Kidney Blood Press Res. 2022. PMID: 36130530 Free PMC article.
References
-
- Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. - PubMed
-
- Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647–662. - PubMed
-
- Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, Smith AJ, Ridley AJ, Ruhrberg C, Gerhardt H, Vanhaesebroeck B. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. - PubMed
-
- Hirsch E, Lembo G, Montrucchio G, Rommel C, Costa C, Barberis L. Signaling through PI3Kgamma: a common platform for leukocyte, platelet and cardiovascular stress sensing. Thromb Haemost. 2006;95:29–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

