FTY720 stimulates 27-hydroxycholesterol production and confers atheroprotective effects in human primary macrophages
- PMID: 20056921
- PMCID: PMC2871287
- DOI: 10.1161/CIRCRESAHA.109.204396
FTY720 stimulates 27-hydroxycholesterol production and confers atheroprotective effects in human primary macrophages
Abstract
Rationale: The synthetic sphingosine analog FTY720 is undergoing clinical trials as an immunomodulatory compound, acting primarily via sphingosine 1-phosphate receptor activation. Sphingolipid and cholesterol homeostasis are closely connected but whether FTY720 affects atherogenesis in humans is not known.
Objective: We examined the effects of FTY720 on the processing of scavenged lipoprotein cholesterol in human primary monocyte-derived macrophages.
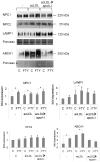
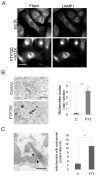
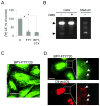
Methods and results: FTY720 did not affect cholesterol uptake but inhibited its delivery to the endoplasmic reticulum, reducing cellular free cholesterol cytotoxicity. This was accompanied by increased levels of Niemann-Pick C1 protein (NPC1) and ATP-binding cassette transporter (ABC)A1 proteins and increased efflux of endosomal cholesterol to apolipoprotein A-I. These effects were not dependent on sphingosine 1-phosphate receptor activation. Instead, FTY720 stimulated the production of 27-hydroxycholesterol, an endogenous ligand of the liver X receptor, leading to liver X receptor-induced upregulation of ABCA1. Fluorescently labeled FTY720 was targeted to late endosomes, and the FTY720-induced upregulation of ABCA1 was NPC1-dependent, but the endosomal exit of FTY720 itself was not.
Conclusions: We conclude that FTY720 decreases cholesterol toxicity in primary human macrophages by reducing the delivery of scavenged lipoprotein cholesterol to the endoplasmic reticulum and facilitating its release to physiological extracellular acceptors. Furthermore, FTY720 stimulates 27-hydroxycholesterol production, providing an explanation for the atheroprotective effects and identifying a novel mechanism by which FTY720 modulates signaling.
Conflict of interest statement
Figures

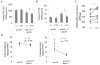



References
-
- Mansoor M, Melendez AJ. Recent trials for FTY720 (fingolimod): a new generation of immunomodulators structurally similar to sphingosine. Rev Recent Clin Trials. 2008;3:62–69. - PubMed
-
- Kihara A, Igarashi Y. Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720. Biochim Biophys Acta. 2008;1781:496–502. - PubMed
-
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. - PubMed
-
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

