Intramyocardial fibroblast myocyte communication
- PMID: 20056945
- PMCID: PMC2805465
- DOI: 10.1161/CIRCRESAHA.109.207456
Intramyocardial fibroblast myocyte communication
Abstract
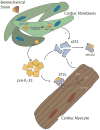
Cardiac fibroblasts are emerging as key components of normal cardiac function, as well as the response to stressors and injury. These most numerous cells of the heart interact with myocytes via paracrine mechanisms, alterations in extracellular matrix homeostasis, and direct cell-cell interactions. It is possible that they are a contributor to the inability of adult myocytes to proliferate and may influence cardiac progenitor biology. Furthering our understanding of how cardiac fibroblasts and myocytes interact may provide an avenue to novel treatments for heart failure prevention. This review discusses the most recent concepts in cardiac fibroblast-myocyte communication and areas of potential future research.
Figures


References
-
- Brilla CG, Maisch B, Weber KT. Myocardial collagen matrix remodelling in arterial hypertension. Eur Heart J. 1992;13(Suppl D):24–32. - PubMed
-
- Weber KT, Brilla CG. Myocardial fibrosis and the renin-angiotensin-aldosterone system. J Cardiovasc Pharmacol. 1992;20(Suppl 1):S48–54. - PubMed
-
- Weber KT, Brilla CG, Campbell SE, Zhou G, Matsubara L, Guarda E. Pathologic hypertrophy with fibrosis: the structural basis for myocardial failure. Blood Press. 1992;1:75–85. - PubMed
-
- Weber KT, Brilla CG, Janicki JS, Reddy HK, Campbell SE. Myocardial fibrosis: role of ventricular systolic pressure, arterial hypertension, and circulating hormones. Basic Res Cardiol. 1991;86(Suppl 3):25–31. - PubMed
-
- Weber KT, Jalil JE, Janicki JS, Pick R. Myocardial collagen remodeling in pressure overload hypertrophy. A case for interstitial heart disease. Am J Hypertens. 1989;2:931–940. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

