When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology
- PMID: 20056956
- PMCID: PMC2808348
- DOI: 10.1093/jnci/djp472
When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology
Abstract
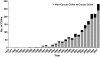
New cancer treatments pose a substantial financial burden on health-care systems, insurers, patients, and society. Cost-utility analyses (CUAs) of cancer-related interventions have received increased attention in the medical literature and are being used to inform reimbursement decisions in many health-care systems. We identified and reviewed 242 cancer-related CUAs published through 2007 and included in the Tufts Medical Center Cost-Effectiveness Analysis Registry (www.cearegistry.org). Leading cancer types studied were breast (36% of studies), colorectal (12%), and hematologic cancers (10%). Studies have examined interventions for tertiary prevention (73% of studies), secondary prevention (19%), and primary prevention (8%). We present league tables by disease categories that consist of a description of the intervention, its comparator, the target population, and the incremental cost-effectiveness ratio. The median reported incremental cost-effectiveness ratios (in 2008 US $) were $27,000 for breast cancer, $22,000 for colorectal cancer, $34,500 for prostate cancer, $32,000 for lung cancer, and $48,000 for hematologic cancers. The results highlight the many opportunities for efficient investment in cancer care across different cancer types and interventions and the many investments that are inefficient. Because we found only modest improvement in the quality of studies, we suggest that journals provide specific guidance for reporting CUA and assure that authors adhere to guidelines for conducting and reporting economic evaluations.
Figures
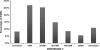
References
-
- Bach PB. Costs of cancer care: a view from the centers for Medicare and Medicaid services. J Clin Oncol. 2007;25(2):187–190. - PubMed
-
- Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626–633. - PubMed
-
- Drummond MF, Mason AR. European perspective on the costs and cost-effectiveness of cancer therapies. J Clin Oncol. 2007;25(2):191–195. - PubMed
-
- Meropol NJ, Schulman KA. Cost of cancer care: issues and implications. J Clin Oncol. 2007;25(2):180–186. - PubMed
-
- Raftery J. NICE and the challenge of cancer drugs. BMJ. 2009;338 b67. - PubMed

