Identification of a novel Bves function: regulation of vesicular transport
- PMID: 20057356
- PMCID: PMC2830705
- DOI: 10.1038/emboj.2009.379
Identification of a novel Bves function: regulation of vesicular transport
Abstract
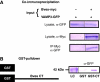
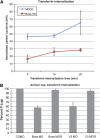
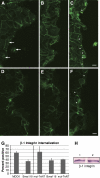
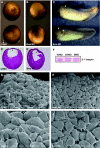
Blood vessel/epicardial substance (Bves) is a transmembrane protein that influences cell adhesion and motility through unknown mechanisms. We have discovered that Bves directly interacts with VAMP3, a SNARE protein that facilitates vesicular transport and specifically recycles transferrin and beta-1-integrin. Two independent assays document that cells expressing a mutated form of Bves are severely impaired in the recycling of these molecules, a phenotype consistent with disruption of VAMP3 function. Using Morpholino knockdown in Xenopus laevis, we demonstrate that elimination of Bves function specifically inhibits transferrin receptor recycling, and results in gastrulation defects previously reported with impaired integrin-dependent cell movements. Kymographic analysis of Bves-depleted primary and cultured cells reveals severe impairment of cell spreading and adhesion on fibronectin, indicative of disruption of integrin-mediated adhesion. Taken together, these data demonstrate that Bves interacts with VAMP3 and facilitates receptor recycling both in vitro and during early development. Thus, this study establishes a newly identified role for Bves in vesicular transport and reveals a novel, broadly applied mechanism governing SNARE protein function.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Bves and NDRG4 regulate directional epicardial cell migration through autocrine extracellular matrix deposition.Mol Biol Cell. 2013 Nov;24(22):3496-510. doi: 10.1091/mbc.E12-07-0539. Epub 2013 Sep 18. Mol Biol Cell. 2013. PMID: 24048452 Free PMC article.
-
Xbves is a regulator of epithelial movement during early Xenopus laevis development.Proc Natl Acad Sci U S A. 2006 Jan 17;103(3):614-9. doi: 10.1073/pnas.0506095103. Epub 2006 Jan 9. Proc Natl Acad Sci U S A. 2006. PMID: 16407138 Free PMC article.
-
Silencing of VAMP3 inhibits cell migration and integrin-mediated adhesion.Biochem Biophys Res Commun. 2009 Feb 27;380(1):65-70. doi: 10.1016/j.bbrc.2009.01.036. Epub 2009 Jan 19. Biochem Biophys Res Commun. 2009. PMID: 19159614 Free PMC article.
-
Bves, a member of the Popeye domain-containing gene family.Dev Dyn. 2006 Mar;235(3):586-93. doi: 10.1002/dvdy.20688. Dev Dyn. 2006. PMID: 16444674 Free PMC article. Review.
-
Blood Vessel Epicardial Substance (BVES) in junctional signaling and cancer.Tissue Barriers. 2018;6(4):1-12. doi: 10.1080/21688370.2018.1499843. Epub 2018 Oct 11. Tissue Barriers. 2018. PMID: 30307367 Free PMC article. Review.
Cited by
-
The cAMP-binding Popdc proteins have a redundant function in the heart.Biochem Soc Trans. 2014 Apr;42(2):295-301. doi: 10.1042/BST20130264. Biochem Soc Trans. 2014. PMID: 24646234 Free PMC article. Review.
-
Helicobacter pylori reduces METTL14-mediated VAMP3 m6A modification and promotes the development of gastric cancer by regulating LC3C-mediated c-Met recycling.Cell Death Discov. 2025 Jan 18;11(1):13. doi: 10.1038/s41420-025-02289-z. Cell Death Discov. 2025. PMID: 39827141 Free PMC article.
-
Mice Lacking the cAMP Effector Protein POPDC1 Show Enhanced Hippocampal Synaptic Plasticity.Cereb Cortex. 2022 Aug 3;32(16):3457-3471. doi: 10.1093/cercor/bhab426. Cereb Cortex. 2022. PMID: 34937090 Free PMC article.
-
Stack-VTP: prediction of vesicle transport proteins based on stacked ensemble classifier and evolutionary information.BMC Bioinformatics. 2023 Apr 7;24(1):137. doi: 10.1186/s12859-023-05257-5. BMC Bioinformatics. 2023. PMID: 37029385 Free PMC article.
-
The Popeye domain containing 2 (popdc2) gene in zebrafish is required for heart and skeletal muscle development.Dev Biol. 2012 Mar 15;363(2):438-50. doi: 10.1016/j.ydbio.2012.01.015. Epub 2012 Jan 28. Dev Biol. 2012. PMID: 22290329 Free PMC article.
References
-
- Andree B, Hillemann T, Kessler-Icekson G, Schmitt-John T, Jockusch H, Arnold HH, Brand T (2000) Isolation and characterization of the novel popeye gene family expressed in skeletal muscle and heart. Dev Biol 223: 371–382 - PubMed
-
- Bhattacharya S, Stewart BA, Niemeyer BA, Burgess RW, McCabe BD, Lin P, Boulianne G, O'Kane CJ, Schwarz TL (2002) Members of the synaptobrevin/vesicle-associated membrane protein (VAMP) family in Drosophila are functionally interchangeable in vivo for neurotransmitter release and cell viability. Proc Natl Acad Sci USA 99: 13867–13872 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

