Genetically encoded optical sensors for monitoring of intracellular chloride and chloride-selective channel activity
- PMID: 20057911
- PMCID: PMC2802328
- DOI: 10.3389/neuro.02.015.2009
Genetically encoded optical sensors for monitoring of intracellular chloride and chloride-selective channel activity
Abstract
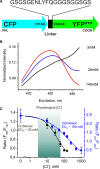
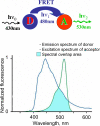
This review briefly discusses the main approaches for monitoring chloride (Cl(-)), the most abundant physiological anion. Noninvasive monitoring of intracellular Cl(-) ([Cl(-)]i) is a challenging task owing to two main difficulties: (i) the low transmembrane ratio for Cl(-), approximately 10:1; and (ii) the small driving force for Cl(-), as the Cl(-) reversal potential (E(Cl)) is usually close to the resting potential of the cells. Thus, for reliable monitoring of intracellular Cl(-), one has to use highly sensitive probes. From several methods for intracellular Cl(-) analysis, genetically encoded chloride indicators represent the most promising tools. Recent achievements in the development of genetically encoded chloride probes are based on the fact that yellow fluorescent protein (YFP) exhibits Cl(-)-sensitivity. YFP-based probes have been successfully used for quantitative analysis of Cl(-) transport in different cells and for high-throughput screening of modulators of Cl(-)-selective channels. Development of a ratiometric genetically encoded probe, Clomeleon, has provided a tool for noninvasive estimation of intracellular Cl(-) concentrations. While the sensitivity of this protein to Cl(-) is low (EC(50) about 160 mM), it has been successfully used for monitoring intracellular Cl(-) in different cell types. Recently a CFP-YFP-based probe with a relatively high sensitivity to Cl(-) (EC(50) about 30 mM) has been developed. This construct, termed Cl-Sensor, allows ratiometric monitoring using the fluorescence excitation ratio. Of particular interest are genetically encoded probes for monitoring of ion channel distribution and activity. A new molecular probe has been constructed by introducing into the cytoplasmic domain of the Cl(-)-selective glycine receptor (GlyR) channel the CFP-YFP-based Cl-Sensor. This construct, termed BioSensor-GlyR, has been successfully expressed in cell lines. The new genetically encoded chloride probes offer means of screening pharmacological agents, analysis of Cl(-) homeostasis and functions of Cl(-)-selective channels under different physiological and pathological conditions.
Keywords: FRET; fluorescent dyes; fluorescent proteins; glycine receptor channel; ion-sensitive microelectrodes; noninvasive monitoring; quinolinium indicators.
Figures


Similar articles
-
Monitoring of chloride and activity of glycine receptor channels using genetically encoded fluorescent sensors.Philos Trans A Math Phys Eng Sci. 2008 Oct 13;366(1880):3445-62. doi: 10.1098/rsta.2008.0133. Philos Trans A Math Phys Eng Sci. 2008. PMID: 18632458
-
Genetically encoded chloride indicator with improved sensitivity.J Neurosci Methods. 2008 May 15;170(1):67-76. doi: 10.1016/j.jneumeth.2007.12.016. Epub 2008 Jan 6. J Neurosci Methods. 2008. PMID: 18279971
-
Calibration and functional analysis of three genetically encoded Cl(-)/pH sensors.Front Mol Neurosci. 2013 Apr 18;6:9. doi: 10.3389/fnmol.2013.00009. eCollection 2013. Front Mol Neurosci. 2013. PMID: 23616745 Free PMC article.
-
Optical Imaging of Brain Activity In Vivo Using Genetically Encoded Probes.In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 1. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 1. PMID: 26844329 Free Books & Documents. Review.
-
Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones.J Physiol. 1987 Apr;385:243-86. doi: 10.1113/jphysiol.1987.sp016493. J Physiol. 1987. PMID: 2443667 Free PMC article. Review.
Cited by
-
Increased intracellular Cl- concentration improves airway epithelial migration by activating the RhoA/ROCK Pathway.Theranostics. 2020 Jul 9;10(19):8528-8540. doi: 10.7150/thno.46002. eCollection 2020. Theranostics. 2020. PMID: 32754261 Free PMC article.
-
Cell viscoelasticity is linked to fluctuations in cell biomass distributions.Sci Rep. 2020 May 4;10(1):7403. doi: 10.1038/s41598-020-64259-y. Sci Rep. 2020. PMID: 32366921 Free PMC article.
-
A noninvasive optical approach for assessing chloride extrusion activity of the K-Cl cotransporter KCC2 in neuronal cells.BMC Neurosci. 2017 Jan 31;18(1):23. doi: 10.1186/s12868-017-0336-5. BMC Neurosci. 2017. PMID: 28143398 Free PMC article.
-
A genetically-encoded chloride and pH sensor for dissociating ion dynamics in the nervous system.Front Cell Neurosci. 2013 Nov 13;7:202. doi: 10.3389/fncel.2013.00202. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24312004 Free PMC article.
-
Engineering the ChlorON Series: Turn-On Fluorescent Protein Sensors for Imaging Labile Chloride in Living Cells.ACS Cent Sci. 2023 Dec 18;10(1):77-86. doi: 10.1021/acscentsci.3c01088. eCollection 2024 Jan 24. ACS Cent Sci. 2023. PMID: 38292617 Free PMC article.
References
-
- Abdulnour-Nakhoul S., Nakhoul N. L., Caymaz-Bor C., Orlando R. C. (2002). Chloride transport in rabbit esophageal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G663–G675 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

