Improved adhesion, growth and maturation of vascular smooth muscle cells on polyethylene grafted with bioactive molecules and carbon particles
- PMID: 20057950
- PMCID: PMC2790113
- DOI: 10.3390/ijms10104352
Improved adhesion, growth and maturation of vascular smooth muscle cells on polyethylene grafted with bioactive molecules and carbon particles
Abstract
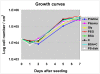
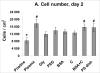
High-density polyethylene (PE) foils were modified by an Ar(+) plasma discharge and subsequent grafting with biomolecules, namely glycine (Gly), polyethylene glycol (PEG), bovine serum albumin (BSA), colloidal carbon particles (C) or BSA and C (BSA + C). As revealed by atomic force microscopy (AFM), goniometry and Rutherford Backscattering Spectroscopy (RBS), the surface chemical structure and surface morphology of PE changed dramatically after plasma treatment. The contact angle decreased for the samples treated by plasma, mainly in relation to the formation of oxygen structures during plasma irradiation. A further decrease in the contact angle was obvious after glycine and PEG grafting. The increase in oxygen concentration after glycine and PEG grafting proved that the two molecules were chemically linked to the plasma-activated surface. Plasma treatment led to ablation of the PE surface layer, thus the surface morphology was changed and the surface roughness was increased. The materials were then seeded with vascular smooth muscle cells (VSMC) derived from rat aorta and incubated in a DMEM medium with fetal bovine serum. Generally, the cells adhered and grew better on modified rather than on unmodified PE samples. Immunofluorescence showed that focal adhesion plaques containing talin, vinculin and paxillin were most apparent in cells on PE grafted with PEG or BSA + C, and the fibres containing alpha-actin, beta-actin or SM1 and SM2 myosins were thicker, more numerous and more brightly stained in the cells on all modified PE samples than on pristine PE. An enzyme-linked immunosorbent assay (ELISA) revealed increased concentrations of focal adhesion proteins talin and vinculin and also a cytoskeletal protein beta-actin in cells on PE modified with BSA + C. A contractile protein alpha-actin was increased in cells on PE grafted with PEG or Gly. These results showed that PE activated with plasma and subsequently grafted with bioactive molecules and colloidal C particles, especially with PEG and BSA + C, promotes the adhesion, proliferation and phenotypic maturation of VSMC.
Keywords: bioactivity; biocompatibility; plasma irradiation; tissue engineering and reconstruction.
Figures











References
-
- Bacakova L, Svorcik V, Rybka V, Micek I, Hnatowicz V, Lisa V, Kocourek F. Adhesion and proliferation of cultured human vascular smooth muscle cells on polystyrene implanted with N+, F+ and Ar+ ions. Biomaterials. 1996;17:1121–1126. - PubMed
-
- Bacakova L, Mares V, Bottone MG, Pellicciari C, Lisa V, Svorcik V. Fluorine-ion-implanted polystyrene improves growth and viability of vascular smooth muscle cells in culture. J. Biomed. Mater. Res. 2000;49:369–379. - PubMed
-
- Bacakova L, Walachova K, Svorcik V, Hnatowicz V. Adhesion and proliferation of rat vascular smooth muscle cells on polyethylene implanted with O+ and C+ ions. J. Biomater. Sci.—Polym. Ed. 2001;12:817–834. - PubMed
-
- Bacakova L, Filova E, Rypacek F, Svorcik V, Stary V. Cell adhesion on artificial materials for tissue engineering. Physiol. Res. 2004;53:S35–S45. - PubMed
-
- Bacakova L, Svorcik V. Cell colonization control by physical and chemical modification of materials. In: Kimura D, editor. Cell Growth Processes: New Research. Nova Science Publishers, Inc.; Hauppauge, NY, USA: 2008. pp. 5–56.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

