Docetaxel versus paclitaxel combined with 5-FU and leucovorin in advanced gastric cancer: combined analysis of two phase II trials
- PMID: 20057964
- PMCID: PMC2802849
- DOI: 10.4143/crt.2009.41.4.196
Docetaxel versus paclitaxel combined with 5-FU and leucovorin in advanced gastric cancer: combined analysis of two phase II trials
Abstract
Purpose: This is an ad hoc analysis of two phase II studies which compared the efficacy and safety of two taxanes (paclitaxel and docetaxel) combined with 5-fluorouracil (5-FU) and leucovorin (LV) in advanced gastric cancer.
Materials and methods: Patients with advanced gastric adenocarcinoma who were untreated or had only received first-line chemotherapy, were treated with either paclitaxel (PFL; 175 mg/m(2)) or docetaxel (DFL; 75 mg/m(2)) on day 1, followed by a bolus of LV (20 mg/m(2) days 1~3) and a 24-hour infusion of 5-FU (1,000 mg/m(2) days 1~3) every 3 weeks. The primary endpoint was overall response rate (ORR) and the secondary endpoint included survival and toxicity.
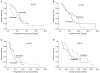
Results: Sixty-six patients received DFL (first-line [n=38]; and second-line [n=28]) and 60 patients received PFL (first-line [n=37]; and second-line [n=23]). The ORRs were not significantly different between the 2 groups (DFL, 26%; PFL, 38%). With a median follow-up of 9.5 months, the progression free survival was 5.2 months (95% confidence interval [CI], 4.2~6.5 months) for DFL and 3.3 months (95% CI, 1.3~5.5 months) for PFL (p=0.17). The overall survival was also comparable between the patients who received DFL and PFL (10.0 months [95% CI, 7.2~12.5 months] and 13.9 months [95% CI, 10.9~19.2 months], respectively; p=0.37). The most frequent grade 3~4 adverse event was neutropenia (DFL, 71%; PFL, 62%). DFL and PFL had different non-hematologic toxicities; specifically, grade >or=3 mucositis (5%) and diarrhea (3%) were common in DFL, while nausea/vomiting (15%) and peripheral neuropathy (5%) were common in PFL.
Conclusion: Thus, the two taxanes had similar efficacy in the treatment of advanced gastric cancer, but different toxicity profiles. Prospective comparative studies are required to further clarify the role of taxanes in the treatment of advanced gastric cancer.
Keywords: Docetaxel; Paclitaxel; Stomach neoplasms.
Figures
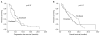
References
-
- Lee JA, Yoon SS, Yang SH, Kim S, Heo DS, Bang YG, et al. FAM versus etoposide, adriamycin, and cisplatin: a random assignment trial in advanced gastric cancer. J Korean Cancer Assoc. 1993;25:461–467.
-
- Thuss-Patience PC, Kretzschmar A, Repp M, Kingreen D, Hennesser D, Micheel S, et al. Docetaxel and continuous-infusion fluorouracil versus epirubicin, cisplatin, and fluorouracil for advanced gastric adenocarcinoma: a randomized phase II study. J Clin Oncol. 2005;23:494–501. - PubMed
-
- Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, et al. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85:295–301. - PubMed
-
- Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999;22:580–586. - PubMed
-
- Bokemeyer C, Lampe CS, Clemens MR, Hartmann JT, Quietzsch D, Forkmann L, et al. A phase II trial of paclitaxel and weekly 24 h infusion of 5-fluorouracil/folinic acid in patients with advanced gastric cancer. Anticancer Drugs. 1997;8:396–399. - PubMed
LinkOut - more resources
Full Text Sources

