The development, optimization and validation of an assay for high throughput antiviral drug screening against Dengue virus
- PMID: 20057980
- PMCID: PMC2802053
The development, optimization and validation of an assay for high throughput antiviral drug screening against Dengue virus
Abstract
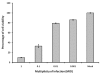
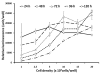
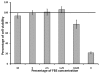
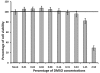
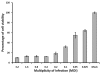
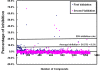
Dengue virus (DENV) is listed as one of the NIAID Category A priority pathogens. Dengue disease is endemic in most tropical countries, with an estimated 2.5 billion people living in areas at risk of DENV infection. Due to the lack of vaccines and antiviral drugs, it is now a huge public health burden around the world. In order to screen large compound libraries for the identification of novel antivirals targeting DENV, it is essential to develop a high throughput screening (HTS) amenable assay. Here, we present the development, optimization and validation of a cytopathic effect-based assay against Dengue virus serotype-2 (DENV-2). The assay conditions, including cell culturing conditions, DMSO tolerance and the multiplicity of infection, were optimized in both 96- and 384-well plates. Assay robustness and reproducibility were determined under the optimized conditions in 96-well plate, including Z'-value of 0.71, signal-to-background ratio of 6.88, coefficient of variation of 6.3% in mock-infected cells and 12.3% in DENV-2 infected cells. This assay was further miniaturized into a 384-well plate format with similar assay robustness and reproducibility comparing with these in the 96-well plate format. This assay was then validated using the LOPAC(1280) compound library, demonstrating its repeatability with comparable assay robustness and reproducibility. This fully developed and validated HTS amenable assay could be used in future studies to screen large compound libraries for the identification of novel antivirals against dengue disease.
Keywords: CPE; Dengue virus; HTS; antiviral; assay development; assay optimization; assay validation; cytopathic effect; high throughput screening.
Figures
Similar articles
-
Assay development and high-throughput antiviral drug screening against Bluetongue virus.Antiviral Res. 2009 Sep;83(3):267-73. doi: 10.1016/j.antiviral.2009.06.004. Epub 2009 Jun 24. Antiviral Res. 2009. PMID: 19559054 Free PMC article.
-
Cell-based antiviral assays for screening and profiling inhibitors against dengue virus.Methods Mol Biol. 2013;1030:185-94. doi: 10.1007/978-1-62703-484-5_15. Methods Mol Biol. 2013. PMID: 23821269
-
Ten years of dengue drug discovery: progress and prospects.Antiviral Res. 2013 Nov;100(2):500-19. doi: 10.1016/j.antiviral.2013.09.013. Epub 2013 Sep 27. Antiviral Res. 2013. PMID: 24076358 Review.
-
Narasin, a novel antiviral compound that blocks dengue virus protein expression.Antivir Ther. 2011;16(8):1203-18. doi: 10.3851/IMP1884. Antivir Ther. 2011. PMID: 22155902
-
Dengue Virus Reporter Replicon is a Valuable Tool for Antiviral Drug Discovery and Analysis of Virus Replication Mechanisms.Viruses. 2016 May 5;8(5):122. doi: 10.3390/v8050122. Viruses. 2016. PMID: 27164125 Free PMC article. Review.
Cited by
-
Antiviral activity of four types of bioflavonoid against dengue virus type-2.Virol J. 2011 Dec 28;8:560. doi: 10.1186/1743-422X-8-560. Virol J. 2011. PMID: 22201648 Free PMC article.
-
High content screening of a kinase-focused library reveals compounds broadly-active against dengue viruses.PLoS Negl Trop Dis. 2013;7(2):e2073. doi: 10.1371/journal.pntd.0002073. Epub 2013 Feb 21. PLoS Negl Trop Dis. 2013. PMID: 23437413 Free PMC article.
-
Implementation of Microfluidics for Antimicrobial Susceptibility Assays: Issues and Optimization Requirements.Front Cell Infect Microbiol. 2020 Sep 17;10:547177. doi: 10.3389/fcimb.2020.547177. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33042872 Free PMC article.
-
In Vitro Activity of Diphenyleneiodonium toward Multidrug-Resistant Helicobacter pylori Strains.Gut Liver. 2017 Sep 15;11(5):648-654. doi: 10.5009/gnl16503. Gut Liver. 2017. PMID: 28750485 Free PMC article.
-
Soluble form of canine transferrin receptor inhibits canine parvovirus infection in vitro and in vivo.Biomed Res Int. 2013;2013:172479. doi: 10.1155/2013/172479. Epub 2013 Sep 8. Biomed Res Int. 2013. PMID: 24089666 Free PMC article.
References
-
- Halstead SB. Dengue virus - Mosquito interactions. Annual Review of Entomology. 2008;53:273–291. - PubMed
-
- WHO. Dengue/dengue hemorrhagic fever. Fact sheet no. 117. 2002. [Accessed June 27, 2009]. 2009; Available at: http://www.who.int/mediacentre/factsheets/fs117/en/index.html.
-
- Ahmed J, Bouloy M, Ergonul O, Fooks A, Paweska J, Chevalier V, Drosten C, Moormann R, Tordo N, Vatansever Z, Calistri P, Estrada-Pena A, Mirazimi A, Unger H, Yin H, Seitzer U. International network for capacity building for the control of emerging viral vector-borne zoonotic diseases: ARBO-ZOONET. Euro Surveill. 2009;14 - PubMed
-
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
