Intranasal delivery of influenza subunit vaccine formulated with GEM particles as an adjuvant
- PMID: 20058113
- PMCID: PMC2844513
- DOI: 10.1208/s12248-009-9168-2
Intranasal delivery of influenza subunit vaccine formulated with GEM particles as an adjuvant
Abstract
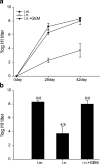
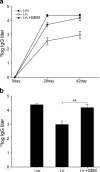
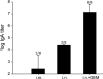
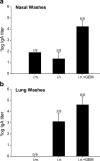
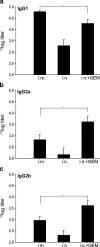
Nasal administration of influenza vaccine has the potential to facilitate influenza control and prevention. However, when administered intranasally (i.n.), commercially available inactivated vaccines only generate systemic and mucosal immune responses if strong adjuvants are used, which are often associated with safety problems. We describe the successful use of a safe adjuvant Gram-positive enhancer matrix (GEM) particles derived from the food-grade bacterium Lactococcus lactis for i.n. vaccination with subunit influenza vaccine in mice. It is shown that simple admixing of the vaccine with the GEM particles results in a strongly enhanced immune response. Already after one booster, the i.n. delivered GEM subunit vaccine resulted in hemagglutination inhibition titers in serum at a level equal to the conventional intramuscular (i.m.) route. Moreover, i.n. immunization with GEM subunit vaccine elicited superior mucosal and Th1 skewed immune responses compared to those induced by i.m. and i.n. administered subunit vaccine alone. In conclusion, GEM particles act as a potent adjuvant for i.n. influenza immunization.
Figures

Similar articles
-
Influenza antigen-sparing by immune stimulation with Gram-positive enhancer matrix (GEM) particles.Vaccine. 2010 Nov 23;28(50):7963-9. doi: 10.1016/j.vaccine.2010.09.066. Epub 2010 Oct 12. Vaccine. 2010. PMID: 20946860
-
A gram-positive enhancer matrix particles vaccine displaying swine influenza virus hemagglutinin protects mice against lethal H1N1 viral challenge.Front Immunol. 2025 Jan 6;15:1432989. doi: 10.3389/fimmu.2024.1432989. eCollection 2024. Front Immunol. 2025. PMID: 39835123 Free PMC article.
-
Gastro-intestinal delivery of influenza subunit vaccine formulation adjuvanted with Gram-positive enhancer matrix (GEM) particles.Eur J Pharm Biopharm. 2010 Nov;76(3):470-4. doi: 10.1016/j.ejpb.2010.08.003. Epub 2010 Aug 16. Eur J Pharm Biopharm. 2010. PMID: 20719246
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
-
A proposal for safety standards for human use of cholera toxin (or Escherichia coli heat-labile enterotoxin) derivatives as an adjuvant of nasal inactivated influenza vaccine.Jpn J Infect Dis. 2000 Jun;53(3):98-106. Jpn J Infect Dis. 2000. PMID: 10957706 Review.
Cited by
-
Lactic acid bacteria--20 years exploring their potential as live vectors for mucosal vaccination.Appl Microbiol Biotechnol. 2015 Apr;99(7):2967-77. doi: 10.1007/s00253-015-6498-0. Epub 2015 Mar 10. Appl Microbiol Biotechnol. 2015. PMID: 25750046 Free PMC article. Review.
-
A novel bacterium-like particles platform displaying antigens by new anchoring proteins induces efficacious immune responses.Front Microbiol. 2024 May 22;15:1395837. doi: 10.3389/fmicb.2024.1395837. eCollection 2024. Front Microbiol. 2024. PMID: 38841059 Free PMC article.
-
The Mechanisms and Application Value of Postbiotics in Caries Prevention and Management.Oral Health Prev Dent. 2024 Sep 12;22:465-478. doi: 10.3290/j.ohpd.b5740317. Oral Health Prev Dent. 2024. PMID: 39264370 Free PMC article. Review.
-
Innovative Mucosal Vaccine Formulations Against Influenza A Virus Infections.Front Immunol. 2019 Jul 17;10:1605. doi: 10.3389/fimmu.2019.01605. eCollection 2019. Front Immunol. 2019. PMID: 31379823 Free PMC article. Review.
-
Bacterium-like particles for efficient immune stimulation of existing vaccines and new subunit vaccines in mucosal applications.Front Immunol. 2013 Sep 17;4:282. doi: 10.3389/fimmu.2013.00282. eCollection 2013. Front Immunol. 2013. PMID: 24062748 Free PMC article.
References
-
- WHO, influenza, fact sheet 211, revised 2003.
-
- Couch RB, Kasel JA, Glezen WP, Cate TR, Six HR, Taber LH, et al. Influenza: its control in persons and populations. J Infect Dis. 1986;153(3):431–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

