Binding and activation of N2O at transition-metal centers: recent mechanistic insights
- PMID: 20058284
- PMCID: PMC2887315
- DOI: 10.1002/anie.200905364
Binding and activation of N2O at transition-metal centers: recent mechanistic insights
Abstract
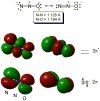
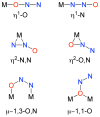
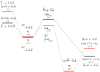
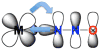
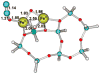
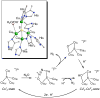
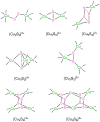
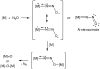
No laughing matter, nitrous oxide's role in stratospheric ozone depletion and as a greenhouse gas has stimulated great interest in developing and understanding its decomposition, particularly through the use of transition-metal promoters. Recent advances in our understanding of the reaction pathways for N(2)O reduction by metal ions in the gas phase and in heterogeneous, homogeneous, and biological catalytic systems have provided provocative ideas about the structure and properties of metal N(2)O adducts and derived intermediates. These ideas are likely to inform efforts to design more effective catalysts for N(2)O remediation.
Figures


Similar articles
-
N2O reduction by the mu4-sulfide-bridged tetranuclear CuZ cluster active site.Angew Chem Int Ed Engl. 2004 Aug 13;43(32):4132-40. doi: 10.1002/anie.200301734. Angew Chem Int Ed Engl. 2004. PMID: 15307074 Review.
-
Oxidation of a [Cu2S] complex by N2O and CO2: insights into a role of tetranuclearity in the CuZ site of nitrous oxide reductase.Chem Commun (Camb). 2018 Jan 25;54(9):1097-1100. doi: 10.1039/c7cc09067f. Chem Commun (Camb). 2018. PMID: 29333559 Free PMC article.
-
Computational Evaluation of Potential Molecular Catalysts for Nitrous Oxide Decomposition.Inorg Chem. 2022 Sep 19;61(37):14591-14605. doi: 10.1021/acs.inorgchem.2c01598. Epub 2022 Sep 6. Inorg Chem. 2022. PMID: 36067530
-
Mechanism of N2O reduction by the mu4-S tetranuclear CuZ cluster of nitrous oxide reductase.J Am Chem Soc. 2006 Jan 11;128(1):278-90. doi: 10.1021/ja055856o. J Am Chem Soc. 2006. PMID: 16390158
-
Nitrous Oxide Metabolism in Nitrate-Reducing Bacteria: Physiology and Regulatory Mechanisms.Adv Microb Physiol. 2016;68:353-432. doi: 10.1016/bs.ampbs.2016.02.007. Epub 2016 Mar 29. Adv Microb Physiol. 2016. PMID: 27134026 Review.
Cited by
-
Mechanistic studies on the addition of hydrogen to iridaepoxide complexes with subsequent elimination of water.Chem Sci. 2016 Feb 1;7(2):921-931. doi: 10.1039/c5sc03575a. Epub 2015 Oct 27. Chem Sci. 2016. PMID: 30155011 Free PMC article.
-
Surface or Internal Hydration - Does It Really Matter?J Am Soc Mass Spectrom. 2023 Mar 1;34(3):337-354. doi: 10.1021/jasms.2c00290. Epub 2023 Feb 6. J Am Soc Mass Spectrom. 2023. PMID: 36744598 Free PMC article. Review.
-
Copper-Promoted Functionalization of Organic Molecules: from Biologically Relevant Cu/O2 Model Systems to Organometallic Transformations.Chem Rev. 2019 Feb 27;119(4):2954-3031. doi: 10.1021/acs.chemrev.8b00368. Epub 2019 Jan 30. Chem Rev. 2019. PMID: 30698952 Free PMC article. Review.
-
Rhodium(I) Pincer Complexes of Nitrous Oxide.Angew Chem Int Ed Engl. 2019 Oct 21;58(43):15295-15298. doi: 10.1002/anie.201908333. Epub 2019 Sep 12. Angew Chem Int Ed Engl. 2019. PMID: 31513331 Free PMC article.
-
Hydrogenation and Hydrosilylation of Nitrous Oxide Homogeneously Catalyzed by a Metal Complex.J Am Chem Soc. 2017 Apr 26;139(16):5720-5723. doi: 10.1021/jacs.7b02124. Epub 2017 Apr 12. J Am Chem Soc. 2017. PMID: 28402635 Free PMC article.
References
-
- Trogler WC. Coord Chem Rev. 1999;187:303–327.
-
- Leont’ev AV, Fomicheva OA, Proskurnina MV, Zefirov NS. Russ Chem Rev. 2001;70:91–104.
-
- 2009 US Greenhouse Gas Inventory Report. Environmental Protection Agency; ( http://tinyurl.com/emissionsreport)
-
- Prather M. Science. 1998;279:1339–1341. - PubMed
-
- Ravishankara AR, Daniel JS, Portmann RW. Science. 2009;326:123–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

