A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors
- PMID: 20058308
- PMCID: PMC3656417
- DOI: 10.1002/cne.22243
A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors
Abstract
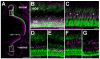
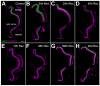
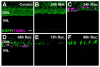
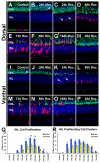
The adult zebrafish retina continuously produces rod photoreceptors from infrequent Müller glial cell division, yielding neuronal progenitor cells that migrate to the outer nuclear layer and become rod precursor cells that are committed to differentiate into rods. Retinal damage models suggested that rod cell death induces regeneration from rod precursor cells, whereas loss of any other retinal neurons activates Müller glia proliferation to produce pluripotent neuronal progenitors that can generate any other neuronal cell type in the retina. We tested this hypothesis by creating two transgenic lines that expressed the E. coli nitroreductase enzyme fused to EGFP (NTR-EGFP) in only rods. Treating transgenic adults with metronidazole resulted in two rod cell death models. First, killing all rods throughout the Tg(zop:nfsB-EGFP)(nt19) retina induced robust Müller glial proliferation, which yielded clusters of neuronal progenitor cells. In contrast, ablating only a subset of rods across the Tg(zop:nfsB-EGFP)(nt20) retina led to rod precursor, but not Müller glial, cell proliferation. We propose that two different criteria determine whether rod cell death will induce a regenerative response from the Müller glia rather than from the resident rod precursor cells in the ONL. First, there must be a large amount of rod cell death to initiate Müller glia proliferation. Second, the rod cell death must be acute, rather than chronic, to stimulate regeneration from the Müller glia. This suggests that the zebrafish retina possesses mechanisms to quantify the amount and timing of rod cell death.
Figures



Similar articles
-
Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration.Exp Eye Res. 2008 Nov;87(5):433-44. doi: 10.1016/j.exer.2008.07.009. Epub 2008 Aug 5. Exp Eye Res. 2008. PMID: 18718467 Free PMC article.
-
β-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina.Neural Dev. 2012 Aug 24;7:30. doi: 10.1186/1749-8104-7-30. Neural Dev. 2012. PMID: 22920725 Free PMC article.
-
The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Müller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina.Exp Eye Res. 2010 Nov;91(5):601-12. doi: 10.1016/j.exer.2010.07.017. Epub 2010 Aug 7. Exp Eye Res. 2010. PMID: 20696157 Free PMC article.
-
Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish.Prog Retin Eye Res. 2014 May;40:94-123. doi: 10.1016/j.preteyeres.2013.12.007. Epub 2014 Jan 8. Prog Retin Eye Res. 2014. PMID: 24412518 Free PMC article. Review.
-
The rod photoreceptor lineage of teleost fish.Prog Retin Eye Res. 2011 Nov;30(6):395-404. doi: 10.1016/j.preteyeres.2011.06.004. Epub 2011 Jun 30. Prog Retin Eye Res. 2011. PMID: 21742053 Free PMC article. Review.
Cited by
-
Using the Tg(nrd:egfp)/albino zebrafish line to characterize in vivo expression of neurod.PLoS One. 2012;7(1):e29128. doi: 10.1371/journal.pone.0029128. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235264 Free PMC article.
-
Loss of Pde6a Induces Rod Outer Segment Shrinkage and Visual Alterations in pde6aQ70X Mutant Zebrafish, a Relevant Model of Retinal Dystrophy.Front Cell Dev Biol. 2021 May 20;9:675517. doi: 10.3389/fcell.2021.675517. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095146 Free PMC article.
-
The nitroreductase system of inducible targeted ablation facilitates cell-specific regenerative studies in zebrafish.Methods. 2013 Aug 15;62(3):232-40. doi: 10.1016/j.ymeth.2013.03.017. Epub 2013 Mar 27. Methods. 2013. PMID: 23542552 Free PMC article.
-
Developing zebrafish disease models for in vivo small molecule screens.Curr Opin Chem Biol. 2019 Jun;50:37-44. doi: 10.1016/j.cbpa.2019.02.005. Epub 2019 Mar 28. Curr Opin Chem Biol. 2019. PMID: 30928773 Free PMC article. Review.
-
Repressing notch signaling and expressing TNFα are sufficient to mimic retinal regeneration by inducing Müller glial proliferation to generate committed progenitor cells.J Neurosci. 2014 Oct 22;34(43):14403-19. doi: 10.1523/JNEUROSCI.0498-14.2014. J Neurosci. 2014. PMID: 25339752 Free PMC article.
References
-
- Bridgewater JA, Knox RJ, Pitts JD, Collins MK, Springer CJ. The bystander effect of the nitroreductase/CB1954 enzyme/ prodrug system is due to a cell-permeable metabolite. Hum Gene Ther. 1997;8:709–717. - PubMed
-
- Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. - PubMed
-
- Edwards DI. The action of metronidazole on DNA. J Antimicrob Chemother. 1977;3:43–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

