Indolyne and aryne distortions and nucleophilic regioselectivites
- PMID: 20058924
- PMCID: PMC2819077
- DOI: 10.1021/ja9098643
Indolyne and aryne distortions and nucleophilic regioselectivites
Abstract
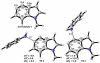
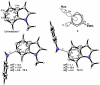
Density functional theory computations reproduce the surprisingly high regioselectivities in nucleophilic additions and cycloadditions to 4,5-indolynes and the low regioselectivities in the reactions of 5,6-indolynes. Transition-state distortion energies control the regioselectivities, activating the 5 and 6 positions over the 4 and 7 positions, leading to high preferences for 5- and 6-substituted products from 4,5- and 6,7-indolynes, respectively. Orbital and electrostatic interactions have only minor effects, producing low regioselectivities in the reactions of 5,6-indolynes. The distortion model predicts high regioselectivities with 6,7-indolynes; these have been verified experimentally. The regioselectivities found with other arynes are explained on the basis of distortion energies that are reflected in reactant geometries.
Figures
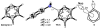
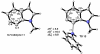
References
-
- Bandini M, Eichholzer A. Angew. Chem. Int. Ed. 2009;48:9608–9644. - PubMed
-
- Tian X, Huters AD, Douglas CJ, Garg NK. Org. Lett. 2009;11:2349–2351. - PubMed
-
- Bronner SM, Bahnck KB, Garg NK. Org. Lett. 2009;11:1007–1010. - PubMed
-
- Buszek KR, Brown N, Luo D. Org. Lett. 2009;11:201–204. - PMC - PubMed
- Brown N, Luo D, Decapo JA, Buszek KR. Tetrahedron Lett. 2009;50:7113–7115. - PMC - PubMed
- Buszek KR, Luo D, Kondrashov M, Brown N, VanderVelde D. Org. Lett. 2007;9:4135–4137. - PubMed
- Brown N, Luo D, VanderVelde D, Yang S, Brassfield A, Buszek KR. Tetrahedron Lett. 2009;50:63–65. - PMC - PubMed
-
-
Also known as deformation energy:Nagase S, Morokuma K. J. Am. Chem. Soc. 1978;100:1666–1672.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

