T cell responses of HIV-infected children after administration of inactivated or live attenuated influenza vaccines
- PMID: 20059397
- PMCID: PMC2858897
- DOI: 10.1089/aid.2009.0163
T cell responses of HIV-infected children after administration of inactivated or live attenuated influenza vaccines
Abstract
Live-attenuated influenza vaccine (LAIV) prevents significantly more cases of influenza in immune-competent children than the trivalent inactivated vaccine (TIV). We compared the T cell responses to LAIV or TIV in HIV-infected children. IFN-gamma-ELISPOT for the three vaccine-contained influenza strains, two mismatched strains, and phytohemagglutinin (PHA), was performed at 0, 4, and 24 weeks postimmunization in 175 HIV-infected children randomly assigned to LAIV or TIV. The contribution of CD8 T cells to the influenza-specific response (CD8-ELISPOT) was evaluated by CD8-cell depletion. CD8 T cells accounted for > or =87% of the total influenza-ELISPOT. At baseline, total influenza-ELISPOT and CD8-ELISPOT values were similar or higher in TIV compared with LAIV recipients. Four and 24 weeks after TIV, total influenza-ELISPOT and CD8-ELISPOT results were significantly lower than baseline results (p < or = 0.001). Responses to PHA also tended to decrease at 4 weeks after TIV (p = 0.06), but rebounded to baseline levels at 24 weeks. Four weeks after LAIV, total influenza-ELISPOT responses to vaccine-contained strains A H3N2 and B significantly decreased. Other ELISPOT values at 4 weeks and all values at 24 weeks were similar to the baseline values. At 4 and 24 weeks, TIV compared to LAIV administration resulted in a significantly greater decrease in influenza-specific ELISPOT values for vaccine-contained influenza A strains (p < or = 0.02). Responses to PHA also tended to decrease more in TIV recipients (p = 0.07). HIV-infected children immunized with TIV had significant and persistent decreases in ELISPOT responses to influenza. LAIV administration suppressed ELISPOT responses less. The clinical significance of these findings deserves further study.
Figures
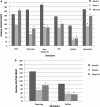

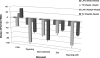
References
-
- Malaspina A. Moir S. Orsega SM, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–1450. - PubMed
-
- Tasker SA. O'Brien WA. Treanor JJ, et al. Effects of influenza vaccination in HIV-infected adults: A double-blind, placebo-controlled trial. Vaccine. 1998;16:1039–1042. - PubMed
-
- King JC., Jr Fast PE. Zangwill KM, et al. Safety, vaccine virus shedding and immunogenicity of trivalent, cold-adapted, live attenuated influenza vaccine administered to human immunodeficiency virus-infected and noninfected children. Pediatr Infect Dis J. 2001;20:1124–1131. - PubMed
-
- King JC. Jr, Treanor J.Fast PE, et al. Comparison of the safety, vaccine virus shedding, and immunogenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults J Infect Dis 2000181725–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

