Prevention and treatment of experimental autoimmune encephalomyelitis with clonotypic CDR3 peptides: CD4(+) Foxp3(+) T-regulatory cells suppress interleukin-2-dependent expansion of myelin basic protein-specific T cells
- PMID: 20059576
- PMCID: PMC2855799
- DOI: 10.1111/j.1365-2567.2009.03218.x
Prevention and treatment of experimental autoimmune encephalomyelitis with clonotypic CDR3 peptides: CD4(+) Foxp3(+) T-regulatory cells suppress interleukin-2-dependent expansion of myelin basic protein-specific T cells
Abstract
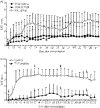
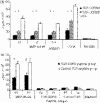
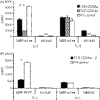
T-cell receptor (TCR)-derived peptides are recognized by the immune system and are capable of modulating autoimmune responses. Using the myelin basic protein (MBP) TCR 1501 transgenic mouse model, we demonstrated that TCR CDR3 peptides from the transgenic TCR can provide a protective effect when therapy is initiated before the induction of experimental autoimmune encephalomyelitis (EAE). More importantly, TCR CDR3 peptide therapy can ameliorate the disease when administered after EAE onset. Concurrent with the therapeutic effects, we observed reduced T-cell proliferation and reduced interleukin-2 (IL-2) levels in response to stimulation with MBP-85-99 peptide in splenocyte cultures from mice receiving TCR CDR3 peptides compared with that of control mice. Moreover, we found that Foxp3(+) CD4 T cells from mice protected with TCR CDR3 peptide are preferentially expanded in the presence of IL-2. This is supportive of a proposed mechanism where Foxp3(+) T-regulatory cells induced by therapy with MBP-85-99 TCR CDR3 peptides limit expansion and the encephalitogenic activity of MBP-85-99-specific T cells by regulating the levels of secreted IL-2.
Figures



References
-
- Olerup O, Hillert J. HLA class II-associated genetic susceptibility to multiple sclerosis: a critical evaluation. Tissue Antigens. 1991;38:1–15. - PubMed
-
- Haines JL, Ter-Minassian M, Bazyk A, et al. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatibility complex. The Multiple Sclerosis Genetics Group. Nat Genet. 1996;13:469–71. - PubMed
-
- Sawcer S, Jones HB, Feakes R, et al. A genome screen in multiple sclerosis reveals susceptibility loci on chromosome 6p21 and 17q22. Nat Genet. 1996;13:464–8. - PubMed
-
- Ebers GC, Kukay K, Bulman DE, et al. A full genome search in multiple sclerosis. Nat Genet. 1996;13:472–6. - PubMed
-
- Hauser SL, Fleischnick E, Weiner HL, Marcus D, Awdeh Z, Yunis EJ, Alper CA. Extended major histocompatibility haplotypes in patients with multiple sclerosis. Neurology. 1989;39:275–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

