Ectodomain shedding of Fcalpha receptor is mediated by ADAM10 and ADAM17
- PMID: 20059578
- PMCID: PMC2855796
- DOI: 10.1111/j.1365-2567.2009.03215.x
Ectodomain shedding of Fcalpha receptor is mediated by ADAM10 and ADAM17
Abstract
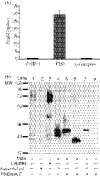
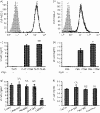
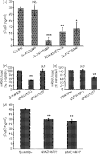
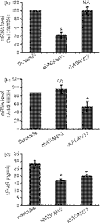
FcalphaR (CD89) plays important roles in immunoglobulin A (IgA)-mediated immune responses. Soluble forms of FcalphaR (sFcalphaR) are found in the culture supernatants of FcalphaR-expressing cells, in human serum and in the serum of FcalphaR transgenic mice, and have been suggested to be produced through a proteolytic process. However, little is known about the mechanism involved in the proteolytic release of sFcalphaR. In this study, we investigated the shedding mechanism of FcalphaR and determined the nature of the proteinase involved in FcalphaR shedding. In chemical inhibitor assays, shedding of FcalphaR was dramatically inhibited by EDTA, EGTA and a broad-spectrum metalloproteinase inhibitor, GM6001, suggesting that a metalloproteinase was responsible for FcalphaR shedding. Overexpression of dominant-negative mutants of ADAM (a disintegrin and metalloproteinase) 10 and ADAM17 markedly inhibited the production of sFcalphaR. Finally, knockdown of both endogenous ADAM10 and endogenous ADAM17 inhibited FcalphaR shedding, demonstrating that ADAM10 and ADAM17 were involved in the shedding of FcalphaR. The characterization of ADAM10 and ADAM17 as sFcalphaR-releasing enzymes provides a novel insight into the molecular mechanism of sFcalphaR production and will help in further elucidation of the physiological and pathological roles of sFcalphaR.
Figures

Similar articles
-
ADAM10 is a principal 'sheddase' of the low-affinity immunoglobulin E receptor CD23.Nat Immunol. 2006 Dec;7(12):1293-8. doi: 10.1038/ni1399. Epub 2006 Oct 29. Nat Immunol. 2006. PMID: 17072319
-
Anticancer chemotherapy inhibits MHC class I-related chain a ectodomain shedding by downregulating ADAM10 expression in hepatocellular carcinoma.Cancer Res. 2009 Oct 15;69(20):8050-7. doi: 10.1158/0008-5472.CAN-09-0789. Epub 2009 Oct 13. Cancer Res. 2009. PMID: 19826051
-
ADAM10 is the major sheddase responsible for the release of membrane-associated meprin A.J Biol Chem. 2014 May 9;289(19):13308-22. doi: 10.1074/jbc.M114.559088. Epub 2014 Mar 24. J Biol Chem. 2014. PMID: 24662289 Free PMC article.
-
The "A Disintegrin And Metalloproteases" ADAM10 and ADAM17: novel drug targets with therapeutic potential?Eur J Cell Biol. 2011 Jun-Jul;90(6-7):527-35. doi: 10.1016/j.ejcb.2010.11.005. Epub 2010 Dec 30. Eur J Cell Biol. 2011. PMID: 21194787 Review.
-
Metalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface molecules.Comb Chem High Throughput Screen. 2005 Mar;8(2):161-71. doi: 10.2174/1386207053258488. Comb Chem High Throughput Screen. 2005. PMID: 15777180 Review.
Cited by
-
Hypothesis: Efficacy of early treatments with some NSAIDs in COVID-19: Might it also depend on their direct and/or indirect zinc chelating ability?Br J Pharmacol. 2023 Feb;180(3):279-286. doi: 10.1111/bph.15989. Epub 2022 Dec 8. Br J Pharmacol. 2023. PMID: 36482040 Free PMC article.
-
Serum Soluble CD89-IgA Complexes Are Elevated in IgA Nephropathy without Immunosuppressant History.Dis Markers. 2020 Jan 16;2020:8393075. doi: 10.1155/2020/8393075. eCollection 2020. Dis Markers. 2020. PMID: 32076466 Free PMC article.
-
SheddomeDB: the ectodomain shedding database for membrane-bound shed markers.BMC Bioinformatics. 2017 Mar 14;18(Suppl 3):42. doi: 10.1186/s12859-017-1465-7. BMC Bioinformatics. 2017. PMID: 28361715 Free PMC article. Review.
-
Repressive effect of primary virus replication on superinfection correlated with gut-derived central memory CD4(+) T cells in SHIV-infected Chinese rhesus macaques.PLoS One. 2013 Sep 2;8(9):e72295. doi: 10.1371/journal.pone.0072295. eCollection 2013. PLoS One. 2013. PMID: 24023734 Free PMC article.
-
Immunomodulatory role of metalloproteinase ADAM17 in tumor development.Front Immunol. 2022 Nov 17;13:1059376. doi: 10.3389/fimmu.2022.1059376. eCollection 2022. Front Immunol. 2022. PMID: 36466812 Free PMC article. Review.
References
-
- Monteiro RC, Cooper MD, Kubagawa H. Molecular heterogeneity of Fcα receptors detected by receptor-specific monoclonal antibodies. J Immunol. 1992;148:1764–70. - PubMed
-
- Geissmann F, Launay P, Pasquier B, Lepelletier Y, Leborgne M, Lehuen A, Brousse N, Monteiro RC. A subset of human dendritic cells expresses IgA Fc receptor (CD89), which mediates internalization and activation upon cross-linking by IgA complexes. J Immunol. 2001;166:346–52. - PubMed
-
- Hamre R, Farstad IN, Brandtzaeg P, Morton HC. Expression and modulation of the human immunoglobulin A Fc receptor (CD89) and the FcR α chain on myeloid cells in blood and tissue. Scand J Immunol. 2003;57:506–16. - PubMed
-
- van Egmond M, van Garderen E, van Spriel AB, et al. FcαRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med. 2000;6:680–5. - PubMed
-
- Monteiro RC, van de Winkel JG. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

