Regulation of eotaxin-3/CCL26 expression in human monocytic cells
- PMID: 20059579
- PMCID: PMC2855795
- DOI: 10.1111/j.1365-2567.2009.03214.x
Regulation of eotaxin-3/CCL26 expression in human monocytic cells
Abstract
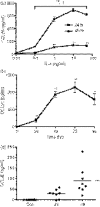
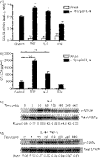
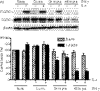
Eotaxin-3/CCL26 is an agonist for chemokine receptor 3 (CCR3) and a natural antagonist for CCR1, CCR2 and CCR5. CCL26 expression by non-haematopoietic cells has been well documented; however, no studies to date have demonstrated CCL26 expression by leucocytes. In this study, we investigated the ability of human monocytic cells to produce CCL26 in response to cytokines. We found that interleukin-4 (IL-4) increased the expression of CCL26 messenger RNA (mRNA) and protein in U937 cells, in human monocytes and in human monocyte-derived macrophages. Tumour necrosis factor-alpha (TNF-alpha) and interleukin-1beta (IL-1beta) alone did not induce CCL26 expression, yet these pro-inflammatory cytokines synergized with IL-4 to increase CCL26 protein expression. Signal transducer and activator of transcription 6 (STAT6) was not affected by costimulation with TNF-alpha, suggesting that the synergy between IL-4 and TNF-alpha occurs at a step downstream of STAT6 activation. Co-incubation of interferon-gamma (IFN-gamma) with IL-4 had no effect on CCL26 protein release. By contrast, pretreatment with IFN-gamma decreased total STAT6 protein, blocked IL-4-mediated STAT6 phosphorylation and decreased IL-4-mediated CCL26 mRNA expression and protein release. These data show that IL-4 and pro-inflammatory cytokines such as TNF-alpha, IL-1beta and IFN-gamma regulate CCL26 synthesis in human monocytic cells, which may be important in regulating monocyte inflammatory responses.
Figures



Similar articles
-
Differential regulation of chemokine expression by Th1 and Th2 cytokines and mechanisms of eotaxin/CCL-11 expression in human airway smooth muscle cells.Int Arch Allergy Immunol. 2007;143 Suppl 1(Suppl 1):84-8. doi: 10.1159/000101412. Epub 2007 May 1. Int Arch Allergy Immunol. 2007. PMID: 17541284 Free PMC article.
-
Activation of eotaxin-3/CCLl26 gene expression in human dermal fibroblasts is mediated by STAT6.J Immunol. 2001 Sep 15;167(6):3216-22. doi: 10.4049/jimmunol.167.6.3216. J Immunol. 2001. PMID: 11544308
-
IFN-gamma-induced SOCS-1 regulates STAT6-dependent eotaxin production triggered by IL-4 and TNF-alpha.Biochem Biophys Res Commun. 2004 Feb 6;314(2):468-75. doi: 10.1016/j.bbrc.2003.12.124. Biochem Biophys Res Commun. 2004. PMID: 14733929
-
Interleukin-4 and interleukin-13 enhance CCL26 production in a human keratinocyte cell line, HaCaT cells.Clin Exp Immunol. 2005 Sep;141(3):459-66. doi: 10.1111/j.1365-2249.2005.02875.x. Clin Exp Immunol. 2005. PMID: 16045735 Free PMC article.
-
Inhibition of IL-4-inducible gene expression in human monocytes by type I and type II interferons.J Leukoc Biol. 1999 Mar;65(3):307-12. doi: 10.1002/jlb.65.3.307. J Leukoc Biol. 1999. PMID: 10080532 Review.
Cited by
-
Pinosylvin Shifts Macrophage Polarization to Support Resolution of Inflammation.Molecules. 2021 May 8;26(9):2772. doi: 10.3390/molecules26092772. Molecules. 2021. PMID: 34066748 Free PMC article.
-
Characterizing the inflammatory response in esophageal mucosal biopsies in children with eosinophilic esophagitis.Clin Transl Immunology. 2016 Jul 1;5(7):e88. doi: 10.1038/cti.2016.30. eCollection 2016 Jul. Clin Transl Immunology. 2016. PMID: 27525061 Free PMC article.
-
The Current and Future of Biomarkers of Immune Related Adverse Events.Rheum Dis Clin North Am. 2024 May;50(2):201-227. doi: 10.1016/j.rdc.2024.01.004. Epub 2024 Mar 12. Rheum Dis Clin North Am. 2024. PMID: 38670721 Free PMC article. Review.
-
Review article: Emerging insights into the epidemiology, pathophysiology, diagnostic and therapeutic aspects of eosinophilic oesophagitis and other eosinophilic gastrointestinal diseases.Aliment Pharmacol Ther. 2024 Feb;59(3):322-340. doi: 10.1111/apt.17845. Epub 2023 Dec 22. Aliment Pharmacol Ther. 2024. PMID: 38135920 Free PMC article. Review.
-
Blood Serum Cytokines in Patients with Subacute Spinal Cord Injury: A Pilot Study to Search for Biomarkers of Injury Severity.Brain Sci. 2021 Mar 4;11(3):322. doi: 10.3390/brainsci11030322. Brain Sci. 2021. PMID: 33806460 Free PMC article.
References
-
- Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams TJ. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993;197:1167–72. - PubMed
-
- Shinkai A, Yoshisue H, Koike M, et al. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4- stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J Immunol. 1999;163:1602–10. - PubMed
-
- De Lucca GV. Recent developments in CCR3 antagonists. Curr Opin Drug Discov Devel. 2006;9:516–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

