Temporal and anatomical sensitivities to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin leading to premature acyclicity with age in rats
- PMID: 20059580
- PMCID: PMC2874621
- DOI: 10.1111/j.1365-2605.2009.01031.x
Temporal and anatomical sensitivities to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin leading to premature acyclicity with age in rats
Abstract
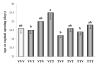
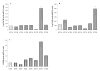
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that mediates diverse dioxin toxicities. While the acute effects of activation of the AhR pathway by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) have been a focus of past study, the role of this pathway in normal physiology and ageing is unclear. The purpose of this study was to identify the portion of the reproductive axis [ovary vs. hypothalamus and pituitary gland (H-H axis)] and the stages of the reproductive lifespan (foetal and early post-natal life vs. adolescence and adulthood) that are particularly sensitive to the effects of TCDD during female reproductive ageing. Adult pregnant Lewis rat dams were dosed with corn oil vehicle or TCDD (50 ng/kg-week by gavage) on days 14 and 21 of gestation and post-natal days 7 and 14 to provide in utero and lactational (IUL) exposure to pups. Female pups (n = 96) were weaned on post-natal day 21 and dosed with TCDD or vehicle weekly. Half of the pups were used as donors for ovary transplantation while the remainder were recipients. Following ovary transplantation, rats (n = 6-8 per group) received weekly TCDD or vehicle again until sacrifice at 8 months of age. Beginning at vaginal opening, reproductive cycles were monitored by vaginal cytology for 10 days each month. Blood samples were collected at 22.00 h on proestrus to measure concentration of 17beta-oestradiol in serum. Real-time PCR was used to determine differences in Cyp1a1, Cyp19a1, Cyp17a1, LH receptor (LHR), FoxA2 and FoxJ1 genes expression between control and remaining groups. IUL exposure of the H-H axis plus adult exposure of the whole body to TCDD significantly delayed puberty in females rats. Data analysis revealed an accelerated onset of acyclicity by 5 months in all groups involving IUL exposure of the developing ovary to TCDD. 17beta-oestradiol was significantly decreased in animals receiving TCDD during IUL exposure of the H-H axis. CYP1a1 expression was markedly greater in the liver than in ovarian tissue and correlated with ongoing TCDD exposure. Aromatase, 17alpha-hydroxylase and LHR gene expressions were largely unchanged (or occasionally elevated) by TCDD. FoxA2 and FoxJ1 mRNAs were similarly of limited value mechanistically, although FoxJ1 was much higher in TTT females (receiving TCDD as donor, recipient and adult). This study reveals a particular sensitivity of the developing ovary to TCDD leading to early loss of reproductive function with age.
Keywords: TCDD; aging; aryl hydrocarbon receptor; ovary; rat.
Figures



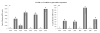
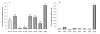
Similar articles
-
Effects of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescence in female Sprague-Dawley rats.Biol Reprod. 2006 Jan;74(1):125-30. doi: 10.1095/biolreprod.105.044396. Epub 2005 Sep 21. Biol Reprod. 2006. PMID: 16177221
-
Effect of chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin in female rats on ovarian gene expression.Reprod Toxicol. 2009 Jul;28(1):32-7. doi: 10.1016/j.reprotox.2009.03.004. Epub 2009 Mar 25. Reprod Toxicol. 2009. PMID: 19490992 Free PMC article.
-
NTP technical report on the toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (Gavage Studies).Natl Toxicol Program Tech Rep Ser. 2006 Apr;(521):4-232. Natl Toxicol Program Tech Rep Ser. 2006. PMID: 16835633
-
NTP Research Report on the CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats: Research Report 9 [Internet].Research Triangle Park (NC): National Toxicology Program; 2018 Sep. Research Triangle Park (NC): National Toxicology Program; 2018 Sep. PMID: 31305969 Free Books & Documents. Review.
-
Reproductive and developmental toxicity of dioxin in fish.Mol Cell Endocrinol. 2012 May 6;354(1-2):121-38. doi: 10.1016/j.mce.2011.09.027. Epub 2011 Sep 21. Mol Cell Endocrinol. 2012. PMID: 21958697 Free PMC article. Review.
Cited by
-
EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals.Endocr Rev. 2015 Dec;36(6):E1-E150. doi: 10.1210/er.2015-1010. Epub 2015 Nov 6. Endocr Rev. 2015. PMID: 26544531 Free PMC article. Review.
-
OXIDATIVE STRESS IN REPRODUCTIVE TOXICOLOGY.Curr Opin Toxicol. 2018 Feb;7:95-101. doi: 10.1016/j.cotox.2017.10.004. Epub 2017 Oct 12. Curr Opin Toxicol. 2018. PMID: 30105313 Free PMC article.
-
Potential protective mechanisms of aryl hydrocarbon receptor (AHR) signaling in benign prostatic hyperplasia.Differentiation. 2011 Nov-Dec;82(4-5):211-9. doi: 10.1016/j.diff.2011.05.011. Differentiation. 2011. PMID: 21684673 Free PMC article. Review.
-
Experimental Evidence of 2,3,7,8-Tetrachlordibenzo-p-Dioxin (TCDD) Transgenerational Effects on Reproductive Health.Int J Mol Sci. 2021 Aug 23;22(16):9091. doi: 10.3390/ijms22169091. Int J Mol Sci. 2021. PMID: 34445797 Free PMC article. Review.
-
Indirect study of the effect of α-tocopherol and acetylsalicylic acid on the mineral composition of bone tissue in the offspring of female rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: long-term observations.RSC Adv. 2019 Mar 11;9(14):8016-8024. doi: 10.1039/c8ra10485a. eCollection 2019 Mar 6. RSC Adv. 2019. PMID: 35547832 Free PMC article.
References
-
- Birnbaum L. Developmental effects of dioxins. In: Korach KS, editor. Reproductive and Developmental Toxicology. Marcel Dekker; New York: 1998. pp. 87–112.
-
- Chaffin CL, Peterson RE, Hutz RJ. In utero and lactational exposure of female Holtzman rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: modulation of the estrogen signal. Biology of Reproduction. 1996;55:62–67. - PubMed
-
- Franczak A, Nynca A, Valdez KE, Mizinga KM, Petroff BK. Effects of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescence in female Sprague-Dawley rats. Biology of Reproduction. 2006;74:125–130. - PubMed
-
- Fugo NW, Butcher RL. Effects of prolonged estrous cycles on reproduction in aged rats. Fertility and Sterility. 1971;22:98–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

