Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching
- PMID: 20059993
- PMCID: PMC2834281
- DOI: 10.1016/j.ydbio.2009.12.033
Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching
Abstract
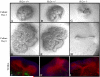
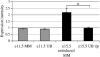
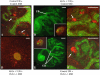
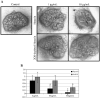
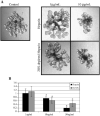
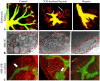
Heparan sulfate proteoglycans (HSPGs) are central modulators of developmental processes likely through their interaction with growth factors, such as GDNF, members of the FGF and TGFbeta superfamilies, EGF receptor ligands and HGF. Absence of the biosynthetic enzyme, heparan sulfate 2-O-sulfotransferase (Hs2st) leads to kidney agenesis. Using a novel combination of in vivo and in vitro approaches, we have reanalyzed the defect in morphogenesis of the Hs2st(-)(/)(-) kidney. Utilizing assays that separately model distinct stages of kidney branching morphogenesis, we found that the Hs2st(-/-) UB is able to undergo branching and induce mesenchymal-to-epithelial transformation when recombined with control MM, and the isolated Hs2st null UB is able to undergo branching morphogenesis in the presence of exogenous soluble pro-branching growth factors when embedded in an extracellular matrix, indicating that the UB is intrinsically competent. This is in contrast to the prevailing view that the defect underlying the renal agenesis phenotype is due to a primary role for 2-O sulfated HS in UB branching. Unexpectedly, the mutant MM was also fully capable of being induced in recombination experiments with wild-type tissue. Thus, both the mutant UB and mutant MM tissue appear competent in and of themselves, but the combination of mutant tissues fails in vivo and, as we show, in organ culture. We hypothesized a 2OS-dependent defect in the mutual inductive process, which could be on either the UB or MM side, since both progenitor tissues express Hs2st. In light of these observations, we specifically examined the role of the HS 2-O sulfation modification on the morphogenetic capacity of the UB and MM individually. We demonstrate that early UB branching morphogenesis is not primarily modulated by factors that depend on the HS 2-O sulfate modification; however, factors that contribute to MM induction are markedly sensitive to the 2-O sulfation modification. These data suggest that key defect in Hs2st null kidneys is the inability of MM to undergo induction either through a failure of mutual induction or a primary failure of MM morphogenesis. This results in normal UB formation but affects either T-shaped UB formation or iterative branching of the T-shaped UB (possibly two separate stages in collecting system development dependent upon HS). We discuss the possibility that a disruption in the interaction between HS and Wnts (e.g. Wnt 9b) may be an important aspect of the observed phenotype. This appears to be the first example of a defect in the MM preventing advancement of early UB branching past the first bifurcation stage, one of the limiting steps in early kidney development.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Growth factor-dependent branching of the ureteric bud is modulated by selective 6-O sulfation of heparan sulfate.Dev Biol. 2011 Aug 1;356(1):19-27. doi: 10.1016/j.ydbio.2011.05.004. Epub 2011 May 11. Dev Biol. 2011. PMID: 21600196 Free PMC article.
-
Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney.Dev Biol. 2004 Aug 15;272(2):310-27. doi: 10.1016/j.ydbio.2004.04.029. Dev Biol. 2004. PMID: 15282150
-
Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney.Dev Biol. 2004 Nov 1;275(1):44-67. doi: 10.1016/j.ydbio.2004.07.022. Dev Biol. 2004. PMID: 15464572
-
Growth factor-heparan sulfate "switches" regulating stages of branching morphogenesis.Pediatr Nephrol. 2014 Apr;29(4):727-35. doi: 10.1007/s00467-013-2725-z. Epub 2014 Feb 2. Pediatr Nephrol. 2014. PMID: 24488503 Review.
-
Heparan sulfate 2-O-sulfotransferase (Hs2st) and mouse development.Glycoconj J. 2002 May-Jun;19(4-5):347-54. doi: 10.1023/A:1025325222530. Glycoconj J. 2002. PMID: 12975615 Review.
Cited by
-
Deciphering functional glycosaminoglycan motifs in development.Curr Opin Struct Biol. 2018 Jun;50:144-154. doi: 10.1016/j.sbi.2018.03.011. Epub 2018 Mar 24. Curr Opin Struct Biol. 2018. PMID: 29579579 Free PMC article. Review.
-
The glomerular basement membrane as a model system to study the bioactivity of heparan sulfate glycosaminoglycans.Microsc Microanal. 2012 Feb;18(1):3-21. doi: 10.1017/S1431927611012682. Microsc Microanal. 2012. PMID: 22258721 Free PMC article. Review.
-
Special Morphological Features at the Interface of the Renal Stem/Progenitor Cell Niche Force to Reinvestigate Transport of Morphogens During Nephron Induction.Biores Open Access. 2016 Jan 1;5(1):49-60. doi: 10.1089/biores.2015.0039. eCollection 2016. Biores Open Access. 2016. PMID: 26862472 Free PMC article. Review.
-
Concepts for a therapeutic prolongation of nephrogenesis in preterm and low-birth-weight babies must correspond to structural-functional properties in the nephrogenic zone.Mol Cell Pediatr. 2017 Dec 7;4(1):12. doi: 10.1186/s40348-017-0078-6. Mol Cell Pediatr. 2017. PMID: 29218481 Free PMC article. Review.
-
The GUDMAP database--an online resource for genitourinary research.Development. 2011 Jul;138(13):2845-53. doi: 10.1242/dev.063594. Development. 2011. PMID: 21652655 Free PMC article.
References
-
- Baeg GH, Selva EM, Goodman RM, Dasgupta R, Perrimon N. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev Biol. 2004;276:89–100. - PubMed
-
- Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, Schwesinger C, Qiao J, Nigam SK. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266:285–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

