Transport of N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine, a metabolite of trichloroethylene, by mouse multidrug resistance associated protein 2 (Mrp2)
- PMID: 20060011
- PMCID: PMC2847015
- DOI: 10.1016/j.taap.2009.12.035
Transport of N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine, a metabolite of trichloroethylene, by mouse multidrug resistance associated protein 2 (Mrp2)
Abstract
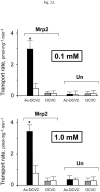
N-acetyl-S-(1,2-dichlorovinyl)-l-cysteine (Ac-DCVC) and S-(1,2-dichlorovinyl)-l-cysteine (DCVC) are the glutathione conjugation pathway metabolites of a common industrial contaminant and potent nephrotoxicant trichloroethylene (TCE). Ac-DCVC and DCVC are accumulated in the renal proximal tubule where they may be secreted into the urine by an unknown apical transporter(s). In this study, we explored the hypothesis that the apical transport of Ac-DCVC and/or DCVC may be mediated by the multidrug resistance associated protein 2 (Mrp2, ABCC2), which is known to mediate proximal tubular apical ATP-dependent transport of glutathione and numerous xenobiotics and endogenous substances conjugated with glutathione. Transport experiments using membrane vesicles prepared from mouse proximal tubule derived cells expressing mouse Mrp2 utilizing ATPase assay and direct measurements of Ac-DCVC/DCVC using liquid chromatography/tandem mass-spectrometry (LC/MS/MS) demonstrated that mouse Mrp2 mediates ATP-dependent transport of Ac-DCVC. Expression of mouse Mrp2 antisense mRNA significantly inhibited the vectorial basolateral to apical transport of Ac-DCVC but not DCVC in mouse proximal tubule derived cells endogenously expressing mouse Mrp2. The results suggest that Mrp2 may be involved in the renal secretion of Ac-DCVC.
Copyright 2009 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures












Similar articles
-
Changes in gene expression in human renal proximal tubule cells exposed to low concentrations of S-(1,2-dichlorovinyl)-l-cysteine, a metabolite of trichloroethylene.Toxicol Appl Pharmacol. 2006 Oct 15;216(2):319-30. doi: 10.1016/j.taap.2006.06.002. Epub 2006 Jun 8. Toxicol Appl Pharmacol. 2006. PMID: 16844155
-
Transport and activation of S-(1,2-dichlorovinyl)-L-cysteine and N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine in rat kidney proximal tubules.Toxicol Appl Pharmacol. 1989 Aug;100(1):51-61. doi: 10.1016/0041-008x(89)90091-4. Toxicol Appl Pharmacol. 1989. PMID: 2763302
-
Characterization of the chemical reactivity and nephrotoxicity of N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine sulfoxide, a potential reactive metabolite of trichloroethylene.Toxicol Appl Pharmacol. 2013 Feb 15;267(1):1-10. doi: 10.1016/j.taap.2012.12.002. Epub 2012 Dec 16. Toxicol Appl Pharmacol. 2013. PMID: 23253325 Free PMC article.
-
N-Acetyl-L-cysteine and aminooxyacetic acid differentially modulate toxicity of the trichloroethylene metabolite S-(1,2-dichlorovinyl)-L-cysteine in human placental villous trophoblast BeWo cells.Toxicology. 2023 Aug 15;495:153611. doi: 10.1016/j.tox.2023.153611. Epub 2023 Aug 5. Toxicology. 2023. PMID: 37544576 Free PMC article.
-
Regulation of expression of the multidrug resistance-associated protein 2 (MRP2) and its role in drug disposition.J Pharmacol Exp Ther. 2002 Aug;302(2):407-15. doi: 10.1124/jpet.102.035014. J Pharmacol Exp Ther. 2002. PMID: 12130697 Review.
Cited by
-
Xenobiotic transporters and kidney injury.Adv Drug Deliv Rev. 2017 Jul 1;116:73-91. doi: 10.1016/j.addr.2017.01.005. Epub 2017 Jan 20. Adv Drug Deliv Rev. 2017. PMID: 28111348 Free PMC article. Review.
-
Harmonization of acronyms for volatile organic compound metabolites using a standardized naming system.Int J Hyg Environ Health. 2021 Jun;235:113749. doi: 10.1016/j.ijheh.2021.113749. Epub 2021 May 4. Int J Hyg Environ Health. 2021. PMID: 33962120 Free PMC article.
-
Editor's Highlight: Comparative Dose-Response Analysis of Liver and Kidney Transcriptomic Effects of Trichloroethylene and Tetrachloroethylene in B6C3F1 Mouse.Toxicol Sci. 2017 Nov 1;160(1):95-110. doi: 10.1093/toxsci/kfx165. Toxicol Sci. 2017. PMID: 28973375 Free PMC article.
References
-
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Trichloroethylene. U.S. Public Health Service, U.S. Department of Health and Human Services; Atlanta, GA: 1993. - PubMed
-
- Anders MW, Dekant W. Glutathione-dependent bioactivation of haloalkenes. Annu Rev Pharmacol Toxicol. 1998;38:501–537. - PubMed
-
- Anders MW, Dekant W, Vamvakas S. Formation and fate of nephrotoxic and cytotoxic glutathione S-conjugates: cysteine conjugate beta-lyase pathway. Adv Pharmacol. 1994;27:115–162. - PubMed
-
- Bencini DA, Wild JR, O'Donovan GA. Linear one-step assay for the determination of orthophosphate. Anal Biochem. 1983;132:254–258. - PubMed
-
- Birner G, Bernauer U, Werner M, Dekant W. Biotransformation, excretion, and nephrotoxicity of haloalkene-derived cysteine S-conjugates. Arch Toxicol. 1997;72:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

