Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: possible pharmacological strategies
- PMID: 20060165
- PMCID: PMC2875138
- DOI: 10.1016/j.ceca.2009.12.009
Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: possible pharmacological strategies
Abstract
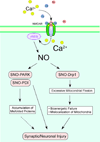
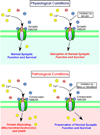
Overactivation of the NMDA-subtype of glutamate receptor is known to trigger excessive calcium influx, contributing to neurodegenerative conditions. Such dysregulation of calcium signaling results in generation of excessive free radicals, including reactive oxygen and nitrogen species (ROS/RNS), including nitric oxide (NO). In turn, we and our colleagues have shown that these free radicals trigger pathological production of misfolded proteins, mitochondrial dysfunction, and apoptotic pathways in neuronal cells. Here, we discuss emerging evidence that excessive calcium-induced NO production can contribute to the accumulation of misfolded proteins, specifically by S-nitrosylation of the ubiquitin E3 ligase, parkin, and the chaperone enzyme for nascent protein folding, protein-disulfide isomerase. Additionally, excessive calcium-induced NO generation leads to the formation of S-nitrosylated dynamin-related protein 1, which causes abnormal mitochondrial fragmentation and resultant synaptic damage. In this review, we also discuss how two novel classes of pharmacological agents hold promise to interrupt these pathological processes. Firstly, the NMDA receptor antagonists, Memantine and NitroMemantine, block excessive extrasynaptic glutamate excitation while maintaining synaptic transmission, thereby limiting excessive calcium influx and production of ROS/RNS. Secondly, therapeutic pro-electrophiles are activated in the face of oxidative insult, thus protecting cells from calcium-induced oxidative stress via the Keap1/Nrf2 transcriptional pathway.
2010 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Inflammatory mediators leading to protein misfolding and uncompetitive/fast off-rate drug therapy for neurodegenerative disorders.Int Rev Neurobiol. 2007;82:1-27. doi: 10.1016/S0074-7742(07)82001-0. Int Rev Neurobiol. 2007. PMID: 17678953 Review.
-
S-nitrosylation of critical protein thiols mediates protein misfolding and mitochondrial dysfunction in neurodegenerative diseases.Antioxid Redox Signal. 2011 Apr 15;14(8):1479-92. doi: 10.1089/ars.2010.3570. Epub 2011 Jan 8. Antioxid Redox Signal. 2011. PMID: 20812868 Free PMC article. Review.
-
Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases.Mol Neurobiol. 2010 Jun;41(2-3):55-72. doi: 10.1007/s12035-010-8113-9. Epub 2010 Mar 25. Mol Neurobiol. 2010. PMID: 20333559 Free PMC article. Review.
-
Redox modulation by S-nitrosylation contributes to protein misfolding, mitochondrial dynamics, and neuronal synaptic damage in neurodegenerative diseases.Cell Death Differ. 2011 Sep;18(9):1478-86. doi: 10.1038/cdd.2011.65. Epub 2011 May 20. Cell Death Differ. 2011. PMID: 21597461 Free PMC article. Review.
-
S-Nitrosylation and uncompetitive/fast off-rate (UFO) drug therapy in neurodegenerative disorders of protein misfolding.Cell Death Differ. 2007 Jul;14(7):1305-14. doi: 10.1038/sj.cdd.4402138. Epub 2007 Apr 13. Cell Death Differ. 2007. PMID: 17431424 Review.
Cited by
-
Neuroprotective effects of methyl-3-O-methyl gallate against sodium fluoride-induced oxidative stress in the brain of rats.Cell Mol Neurobiol. 2013 Mar;33(2):261-7. doi: 10.1007/s10571-012-9893-4. Epub 2012 Nov 29. Cell Mol Neurobiol. 2013. PMID: 23192563 Free PMC article.
-
Understanding chronic inflammation: couplings between cytokines, ROS, NO, Cai 2+, HIF-1α, Nrf2 and autophagy.Front Immunol. 2025 Apr 8;16:1558263. doi: 10.3389/fimmu.2025.1558263. eCollection 2025. Front Immunol. 2025. PMID: 40264757 Free PMC article. Review.
-
Lysosomal disruption preferentially targets acute myeloid leukemia cells and progenitors.J Clin Invest. 2013 Jan;123(1):315-28. doi: 10.1172/JCI64180. Epub 2012 Dec 3. J Clin Invest. 2013. PMID: 23202731 Free PMC article. Clinical Trial.
-
Parkia biglobosa improves mitochondrial functioning and protects against neurotoxic agents in rat brain hippocampal slices.Biomed Res Int. 2014;2014:326290. doi: 10.1155/2014/326290. Epub 2014 Aug 10. Biomed Res Int. 2014. PMID: 25177688 Free PMC article.
-
The PI3K/Akt signaling axis in Alzheimer's disease: a valuable target to stimulate or suppress?Cell Stress Chaperones. 2021 Nov;26(6):871-887. doi: 10.1007/s12192-021-01231-3. Epub 2021 Aug 13. Cell Stress Chaperones. 2021. PMID: 34386944 Free PMC article. Review.
References
-
- Beal MF. Experimental models of Parkinson's disease. Nat Rev Neurosci. 2001;2:325–334. - PubMed
-
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. - PubMed
-
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. - PubMed
-
- Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. - PubMed
-
- Lipton SA, Choi YB, Pan ZH, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

