The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation
- PMID: 20060329
- PMCID: PMC3015141
- DOI: 10.1016/j.immuni.2009.12.003
The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation
Abstract
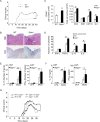
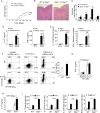
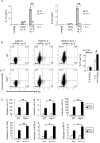
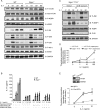
Interleukin-1 (IL-1)-mediated signaling in T cells is essential for T helper 17 (Th17) cell differentiation. We showed here that SIGIRR, a negative regulator of IL-1 receptor and Toll-like receptor signaling, was induced during Th17 cell lineage commitment and governed Th17 cell differentiation and expansion through its inhibitory effects on IL-1 signaling. The absence of SIGIRR in T cells resulted in increased Th17 cell polarization in vivo upon myelin oligodendrocyte glycoprotein (MOG(35-55)) peptide immunization. Recombinant IL-1 promoted a marked increase in the proliferation of SIGIRR-deficient T cells under an in vitro Th17 cell-polarization condition. Importantly, we detected increased IL-1-induced phosphorylation of JNK and mTOR kinase in SIGIRR-deficient Th17 cells compared to wild-type Th17 cells. IL-1-induced proliferation was abolished in mTOR-deficient Th17 cells, indicating the essential role of mTOR activation. Our results demonstrate an important mechanism by which SIGIRR controls Th17 cell expansion and effector function through the IL-1-induced mTOR signaling pathway.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Andoh A, Yasui H, Inatomi O, Zhang Z, Deguchi Y, Hata K, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-17 augments tumor necrosis factor-alpha-induced granulocyte and granulocyte/macrophage colony-stimulating factor release from human colonic myofibroblasts. J. Gastroenterol. 2005;40:802–810. - PubMed
-
- Bamba S, Andoh A, Yasui H, Araki Y, Bamba T, Fujiyama Y. Matrix metalloproteinase-3 secretion from human colonic subepithelial myofibroblasts: role of interleukin-17. J. Gastroenterol. 2003;38:548–554. - PubMed
-
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
-
- Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D'Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, Puccetti P, Mantovani A, Romani L. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J. Immunol. 2008;180:4022–4031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

