The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin
- PMID: 20060330
- PMCID: PMC5836496
- DOI: 10.1016/j.immuni.2009.10.010
The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin
Abstract
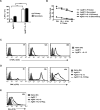
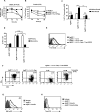
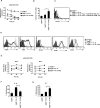
The mechanisms underpinning integration of instructions that program naive CD8+ T cells for effector and/or memory differentiation are not well understood. Herein, we demonstrate that interleukin-12 (IL-12) enhanced and sustained antigen and costimulatory molecule (B7.1)-induced mTOR kinase activity in naive CD8+ (OT-I) T cells via phosphoinositide 3-kinase and STAT4 transcription factor pathways. Blocking mTOR activity by rapamycin reversed IL-12-induced effector functions because of loss of persistent expression of the transcription factor T-bet. Rapamycin treatment of IL-12-conditioned OT-I cells promoted persistent Eomesodermin expression and produced memory cell precursors that demonstrated enhanced sustenance and antigen-recall responses upon adoptive transfer. The memory cell precursors showed greater tumor efficacy than IL-12-conditioned effector OT-I cells. These results identify mTOR as the central regulator of transcriptional programs that determine effector and/or memory cell fates in CD8+ T cells. Targeting mTOR activity offers new opportunities to regulate CD8+ T cell-mediated immunity.
Copyright 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
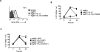


References
-
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. - PubMed
-
- Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. - PubMed
-
- Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

