Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression
- PMID: 20060370
- PMCID: PMC5527897
- DOI: 10.1016/j.molonc.2009.12.001
Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression
Abstract
Glycosylation is the stepwise procedure of covalent attachment of oligosaccharide chains to proteins or lipids, and alterations in this process have been associated with malignant transformation. Simultaneous analysis of the expression of all glycan-related genes clearly gives the advantage of enabling a comprehensive view of the genetic background of the glycobiological changes in cancer cells. Studies focusing on the expression of the whole glycome have now become possible, which prompted us to review the present knowledge on glycosylation in relation to breast cancer diagnosis and progression, in the light of available expression data from tumors and breast tissue of healthy individuals. We used various data resources to select a set of 419 functionally relevant genes involved in synthesis, degradation and binding of N-linked and O-linked glycans, Lewis antigens, glycosaminoglycans (chondroitin, heparin and keratan sulfate in addition to hyaluronan) and glycosphingolipids. Such glycans are involved in a number of processes relevant to carcinogenesis, including regulation of growth factors/growth factor receptors, cell-cell adhesion and motility as well as immune system modulation. Expression analysis of these glycan-related genes revealed that mRNA levels for many of them differ significantly between normal and malignant breast tissue. An associative analysis of these genes in the context of current knowledge of their function in protein glycosylation and connection(s) to cancer indicated that synthesis, degradation and adhesion mediated by glycans may be altered drastically in mammary carcinomas. Although further analysis is needed to assess how changes in mRNA levels of glycan genes influence a cell's glycome and the precise role that such altered glycan structures play in the pathogenesis of the disease, lessons drawn from this study may help in determining directions for future research in the rapidly-developing field of glycobiology.
Copyright 2009 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

 –
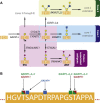
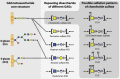
– ) influenced by various glycosylation types are indicated;
) influenced by various glycosylation types are indicated;  growth receptors (especially EGFR and TβR) are influenced by N‐glycosylation in concert with galectins;
growth receptors (especially EGFR and TβR) are influenced by N‐glycosylation in concert with galectins;  growth factors and other signaling molecules may have elevated concentrations, filtered or sequestered by glycosaminoglycans and O‐glycosylated mucins;
growth factors and other signaling molecules may have elevated concentrations, filtered or sequestered by glycosaminoglycans and O‐glycosylated mucins;  cell‐cell adhesion might be mediated either directly by for example glycosynapses consisting mainly of glycosphingolipids ‐ or, more importantly, indirectly by modulation of integrins and cadherins by N‐linked glycosylation;
cell‐cell adhesion might be mediated either directly by for example glycosynapses consisting mainly of glycosphingolipids ‐ or, more importantly, indirectly by modulation of integrins and cadherins by N‐linked glycosylation;  O‐glycosylated mucins, both secreted and membrane‐bound, may constitute a physical barrier or act on specific leukocyte receptors thereby modulating immune system response towards the malignant cells;
O‐glycosylated mucins, both secreted and membrane‐bound, may constitute a physical barrier or act on specific leukocyte receptors thereby modulating immune system response towards the malignant cells;  N‐linked glycosylation may enhance motility of transformed cells by regulating integrin functionality;
N‐linked glycosylation may enhance motility of transformed cells by regulating integrin functionality;  adhesion to endothelium can be mediated by a number of mechanisms, including binding of Lewis antigens by endothelial selectins.
adhesion to endothelium can be mediated by a number of mechanisms, including binding of Lewis antigens by endothelial selectins.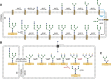


References
-
- Ang, L.C. , Zhang, Y. , Cao, L. , Yang, B.L. , Young, B. , Kiani, C. , Lee, V. , Allan, K. , Yang, B.B. , 1999. Versican enhances locomotion of astrocytoma cells and reduces cell adhesion through its G1 domain. J. Neuropathol. Exp. Neurol. 58, 597–605. - PubMed
-
- Angulo, J. , Ojeda, R. , de Paz, J.L. , Lucas, R. , Nieto, P.M. , Lozano, R.M. , Redondo-Horcajo, M. , Gimenez-Gallego, G. , Martin-Lomas, M. , 2004. The activation of fibroblast growth factors (FGFs) by glycosaminoglycans: influence of the sulfation pattern on the biological activity of FGF-1. Chembiochem. 5, 55–61. - PubMed
-
- Bennett, E.P. , Hassan, H. , Hollingsworth, M.A. , Clausen, H. , 1999. A novel human UDP-N-acetyl-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase, GalNAc-T7, with specificity for partial GalNAc-glycosylated acceptor substrates. FEBS Lett. 460, 226–230. - PubMed
-
- Bennett, E.P. , Hassan, H. , Mandel, U. , Hollingsworth, M.A. , Akisawa, N. , Ikematsu, Y. , Merkx, G. , van Kessel, A.G. , Olofsson, S. , Clausen, H. , 1999. Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J. Biol. Chem. 274, 25362–25370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

