ATR preferentially interacts with common fragile site FRA3B and the binding requires its kinase activity in response to aphidicolin treatment
- PMID: 20060399
- PMCID: PMC2834799
- DOI: 10.1016/j.mrfmmm.2009.12.012
ATR preferentially interacts with common fragile site FRA3B and the binding requires its kinase activity in response to aphidicolin treatment
Abstract
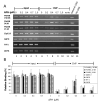
The instability of common fragile sites (CFSs) contributes to the development of a variety of cancers. The ATR-dependent DNA damage checkpoint pathway has been implicated in maintaining CFS stability, but the mechanism is incompletely understood. The goal of our study was to elucidate the action of the ATR protein in the CFS-specific ATR-dependent checkpoint response. Using a chromatin immunoprecipitation assay, we demonstrated that ATR protein preferentially binds (directly or through complexes) to fragile site FRA3B as compared to non-fragile site regions, under conditions of mild replication stress. Interestingly, the amount of ATR protein that bound to three regions of FRA3B peaked at 0.4microM aphidicolin (APH) treatment and decreased again at higher concentrations of APH. The total amounts of cellular ATR and several ATR-interacting proteins remained unchanged, suggesting that ATR binding to the fragile site is guided initially by the level of replication stress signals generated at FRA3B due to APH treatment and then sequestered from FRA3B regions by successive signals from other non-fragile site regions, which are produced at the higher concentrations of APH. This decrease in ATR binding to fragile site FRA3B at the higher concentrations of APH may account for the increasing number of chromosome gaps and breaks observed under the same conditions. Furthermore, inhibition of ATR kinase activity by treatment with 2-aminopurine (2-AP) or by over-expression of a kinase-dead ATR mutant showed that the kinase activity is required for the binding of ATR to fragile DNAs in response to APH treatment. Our results provide novel insight into the mechanism for the regulation of fragile site stability by ATR.
Keywords: ATR; FRA3B; Fragile site.
Copyright 2010 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures


References
-
- Casper AM, Nghiem P, Arlt MF, Glover TW. ATR regulates fragile site stability. Cell. 2002;111:779–789. - PubMed
-
- Durkin SG, Arlt MF, Howlett NG, Glover TW. Depletion of CHK1, but not CHK2, induces chromosomal instability and breaks at common fragile sites. Oncogene. 2006;25:4381–4388. - PubMed
-
- Howlett NG, Taniguchi T, Durkin SG, D'Andrea AD, Glover TW. The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Hum Mol Genet. 2005;14:693–701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

