The inflammasomes: mechanisms of activation and function
- PMID: 20060699
- PMCID: PMC2844336
- DOI: 10.1016/j.coi.2009.12.004
The inflammasomes: mechanisms of activation and function
Abstract
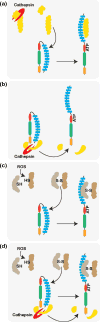
In response to injurious or infectious agents caspase-1 activating multiprotein complexes, termed inflammasomes, assemble in the cytoplasm of cells. Activated caspase-1 cleaves the proforms of the interleukin-1 cytokine family members leading to their activation and secretion. The IL-1 family cytokines have multiple proinflammatory activities implicating them in the pathogenesis of many inflammatory diseases. While defined ligands have been identified for the NLRP1, IPAF, and AIM2 inflammasomes, little is known about the activation mechanisms of the NLRP3 inflammasome. Numerous different molecular entities, such as various crystals, pore-forming toxins, or extracellular ATP can trigger the NLRP3 inflammasome. Recent work proposes that NLRP3 is activated indirectly by host factors that are generated in response to NLRP3 triggers.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
References
-
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. - PubMed
-
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. - PubMed
-
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. - PubMed
-
- Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci U S A. 2007;104:8041–8046. First description of ATP binding to NLRP3 and the functional relevance for NLRP3 activation. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

