Large conformational changes in proteins: signaling and other functions
- PMID: 20060708
- PMCID: PMC2866511
- DOI: 10.1016/j.sbi.2009.12.004
Large conformational changes in proteins: signaling and other functions
Abstract
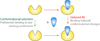
Guanine and adenine nucleotide triphosphatases, such as Ras proteins and protein kinases, undergo large conformational changes upon ligand binding in the course of their functions. New computer simulation methods have combined with experimental studies to deepen our understanding of these phenomena. In particular, a 'conformational selection' picture is emerging, where alterations in the relative populations of pre-existing conformations can best describe the conformational switching activity of these important proteins.
Figures
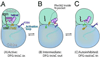
References
-
- Vetter IR, Wittinghofer A. Nucleoside triphosphate-binding proteins: different scaffolds to achieve phosphoryl transfer. Q Rev Biophys. 1999;32:1–56. - PubMed
-
- Frauenfelder H, Sligar SG, Wolynes PG. The energy landscapes and motions of proteins. Science. 1991;254:1598–1603. - PubMed
-
- McCammon JA. Computer-aided molecular design. Science. 1987;238:486–491. - PubMed
-
- Karplus M, McCammon JA. Dynamics of proteins: elements and function. Annu Rev Biochem. 1983;52:263–300. - PubMed
-
- Berendsen HJ, Hayward S. Collective protein dynamics in relation to function. Curr Opin Struct Biol. 2000;10:165–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

