The Foxc2 transcription factor regulates tumor angiogenesis
- PMID: 20060810
- PMCID: PMC2822046
- DOI: 10.1016/j.bbrc.2010.01.015
The Foxc2 transcription factor regulates tumor angiogenesis
Abstract
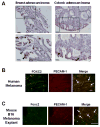
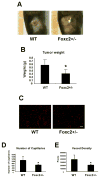
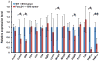
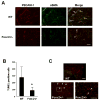
The Forkhead/Fox transcription factor Foxc2 is a critical regulator of vascular development. However, the role of Foxc2 in pathological angiogenesis in cancer remains unknown. Here we show that FoxC2 is highly expressed in human breast and colonic tumors and in the tumor endothelium in human and mouse melanomas. Using the B16 melanoma tumor model, we investigated the function of Foxc2 in tumor angiogenesis. After subcutaneous injection of B16 melanoma cells, primary tumor growth as well as neovascularization was markedly reduced in mice lacking one copy of the Foxc2 gene (Foxc2+/-). Consistently, expression levels of several angiogenic factors, including vascular endothelial growth factor (Vegf), matrix metallopeptidase 2 (Mmp2), and platelet-derived growth factor-B (Pdgfb), were significantly decreased in B16 tumors grown in Foxc2+/- mice, and tumor blood vessels formed in Foxc2+/- mice showed reduced coverage of mural cells and endothelial cell apoptosis. In addition, the tumor tissue in Foxc2+/- mice had an accumulation of necrotic cells. Taken together, these findings demonstrate that haplodeficiency of Foxc2 results in impaired formation of tumor blood vessels as well as reduced tumor growth and thereby provide evidence that Foxc2 is critical for tumor development and angiogenesis.
2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The Role of FoxC2 Transcription Factor in Tumor Angiogenesis.J Oncol. 2012;2012:204593. doi: 10.1155/2012/204593. Epub 2011 Nov 16. J Oncol. 2012. PMID: 22174714 Free PMC article.
-
Foxc2 transcription factor: a newly described regulator of angiogenesis.Trends Cardiovasc Med. 2008 Aug;18(6):224-8. doi: 10.1016/j.tcm.2008.11.003. Trends Cardiovasc Med. 2008. PMID: 19185813 Free PMC article. Review.
-
Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression.Mol Carcinog. 2015 Sep;54(9):926-34. doi: 10.1002/mc.22153. Epub 2014 Apr 2. Mol Carcinog. 2015. PMID: 24700685 Free PMC article.
-
Amino acid transporter LAT1 in tumor-associated vascular endothelium promotes angiogenesis by regulating cell proliferation and VEGF-A-dependent mTORC1 activation.J Exp Clin Cancer Res. 2020 Nov 30;39(1):266. doi: 10.1186/s13046-020-01762-0. J Exp Clin Cancer Res. 2020. PMID: 33256804 Free PMC article.
-
The role of novel prognostic markers PROX1 and FOXC2 in carcinogenesis of oral squamous cell carcinoma.J Exp Ther Oncol. 2018 May;12(3):171-184. J Exp Ther Oncol. 2018. PMID: 29790306 Review.
Cited by
-
Tumor lymphangiogenesis as a potential therapeutic target.J Oncol. 2012;2012:204946. doi: 10.1155/2012/204946. Epub 2012 Feb 16. J Oncol. 2012. PMID: 22481918 Free PMC article.
-
Generation of conditional alleles for Foxc1 and Foxc2 in mice.Genesis. 2012 Oct;50(10):766-74. doi: 10.1002/dvg.22036. Epub 2012 May 14. Genesis. 2012. PMID: 22522965 Free PMC article.
-
Molecular expression of Forkhead Box C2 gene (FOXC2) and Prospero homeobox gene (PROX-1) in oral squamous carcinoma and their correlation with clinicopathological parameters: A prospective cohort study.J Oral Maxillofac Pathol. 2024 Apr-Jun;28(2):216-225. doi: 10.4103/jomfp.jomfp_394_23. Epub 2024 Jul 11. J Oral Maxillofac Pathol. 2024. PMID: 39157851 Free PMC article.
-
Correlation of Forkhead Box c2 with subtypes and invasive ability of invasive breast cancer.J Huazhong Univ Sci Technolog Med Sci. 2014 Dec;34(6):896-901. doi: 10.1007/s11596-014-1370-5. Epub 2014 Dec 6. J Huazhong Univ Sci Technolog Med Sci. 2014. PMID: 25480587
-
Expression of FOXC2 in renal cell carcinoma and its relationship to clinical pathological features.Int J Clin Exp Med. 2015 Aug 15;8(8):13388-92. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26550271 Free PMC article.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
-
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. - PubMed
-
- McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–725. - PubMed
-
- Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

