Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain
- PMID: 20060879
- PMCID: PMC2888630
- DOI: 10.1016/j.bbagen.2009.12.010
Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain
Abstract
Background: The mechanisms through which estrogens modulate neuronal physiology, brain morphology, and behavior in recent years have proven to be far more complex than previously thought. For example, a second nuclear estrogen receptor has been identified, a new family of coregulatory proteins regulating steroid-dependent gene transcriptions was discovered and, finally, it has become clear that estrogens have surprisingly rapid effects based on their actions on cell membranes, which in turn result in the modulation of intracellular signaling cascades.
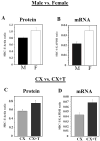
Scope of review: This paper presents a selective review of new findings in this area related to work in our laboratories, focusing on the role of estrogens in the activation of male sexual behavior. Two separate topics are considered. We first discuss functions of the steroid receptor coactivator-1 (SRC-1) that has emerged as a key limiting factor for behavioral effects of estradiol. Knocking-down its expression by antisense oligonucleotides drastically inhibits male-typical sexual behaviors. Secondly, we describe rapid regulations of brain estradiol production by calcium-dependent phosphorylations of the aromatase enzyme, themselves under the control of neurotransmitter activity.
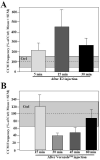
Major conclusions: These rapid changes in estrogen bioavailability have clear behavioral consequences. Increases or decreases in estradiol concentrations respectively obtained by an acute injection of estradiol itself or of an aromatase inhibitor lead within 15-30 min to parallel changes in sexual behavior frequencies.
General significance: These new controls of estrogen action offer a vast array of possibilities for discrete local controls of estrogen action. They also represent a formidable challenge for neuroendocrinologists trying to obtain an integrated view of brain function in relation to behavior.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Sexual behavior activity tracks rapid changes in brain estrogen concentrations.J Neurosci. 2007 Jun 13;27(24):6563-72. doi: 10.1523/JNEUROSCI.1797-07.2007. J Neurosci. 2007. PMID: 17567817 Free PMC article.
-
Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action.Physiol Behav. 2004 Nov 15;83(2):247-70. doi: 10.1016/j.physbeh.2004.08.025. Physiol Behav. 2004. PMID: 15488543 Review.
-
Multiple mechanisms control brain aromatase activity at the genomic and non-genomic level.J Steroid Biochem Mol Biol. 2003 Sep;86(3-5):367-79. doi: 10.1016/s0960-0760(03)00346-7. J Steroid Biochem Mol Biol. 2003. PMID: 14623533 Review.
-
Phosphorylation processes mediate rapid changes of brain aromatase activity.J Steroid Biochem Mol Biol. 2001 Dec;79(1-5):261-77. doi: 10.1016/s0960-0760(01)00143-1. J Steroid Biochem Mol Biol. 2001. PMID: 11850233 Review.
-
Targeting steroid receptor coactivator-1 expression with locked nucleic acids antisense reveals different thresholds for the hormonal regulation of male sexual behavior in relation to aromatase activity and protein expression.Behav Brain Res. 2006 Sep 25;172(2):333-43. doi: 10.1016/j.bbr.2006.05.023. Epub 2006 Jun 23. Behav Brain Res. 2006. PMID: 16797739
Cited by
-
Rapid Effects of Estradiol on Aggression in Birds and Mice: The Fast and the Furious.Integr Comp Biol. 2015 Aug;55(2):281-93. doi: 10.1093/icb/icv048. Epub 2015 May 16. Integr Comp Biol. 2015. PMID: 25980562 Free PMC article. Review.
-
Cell-Based High-Throughput Screening for Aromatase Inhibitors in the Tox21 10K Library.Toxicol Sci. 2015 Oct;147(2):446-57. doi: 10.1093/toxsci/kfv141. Epub 2015 Jul 3. Toxicol Sci. 2015. PMID: 26141389 Free PMC article.
-
The expression of select genes necessary for membrane-associated estrogen receptor signaling differ by sex in adult rat hippocampus.Steroids. 2019 Feb;142:21-27. doi: 10.1016/j.steroids.2017.09.012. Epub 2017 Sep 28. Steroids. 2019. PMID: 28962849 Free PMC article.
-
The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health.Int J Mol Sci. 2022 Oct 8;23(19):11952. doi: 10.3390/ijms231911952. Int J Mol Sci. 2022. PMID: 36233256 Free PMC article. Review.
-
Sex differences in neurodevelopmental abnormalities caused by early-life anaesthesia exposure: a narrative review.Br J Anaesth. 2020 Mar;124(3):e81-e91. doi: 10.1016/j.bja.2019.12.032. Epub 2020 Jan 21. Br J Anaesth. 2020. PMID: 31980157 Free PMC article. Review.
References
-
- Soma KK, Scotti MA, Newman AE, Charlier TD, Demas GE. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 2008;29:476–489. - PubMed
-
- Celotti F, Massa R, Martini L. Metabolism of sex steroids in the central nervous system. In: DeGroot LJ, editor. Endocrinology. Grune & Stratton; New York: 1979. pp. 41–53.
-
- Martini L, Celotti F, Lechuga MJ, Melcangi RC, Motta M, Negri-Cesi P, Poletti A, Zoppi S. Androgen metabolism in different target tissues. Ann N Y Acad Sci. 1990;595:184–198. - PubMed
-
- Balthazart J. Steroid metabolism and the activation of social behavior. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology. Vol. 3. Springer Verlag; Berlin: 1989. pp. 105–159.
-
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975;31:295–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

