Caspase-1 recognizes extended cleavage sites in its natural substrates
- PMID: 20060974
- PMCID: PMC2917068
- DOI: 10.1016/j.atherosclerosis.2009.12.017
Caspase-1 recognizes extended cleavage sites in its natural substrates
Abstract
Objective: The preferred amino acids in the proteolytic sites have been considered to be similar between caspase-1 and caspase-9, which do not support their differential functions in inflammatory pyroptosis and apoptosis. We attempted to solve this problem.
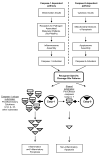
Methods: We analyzed the flanking 20 amino acid residues in the cleavage sites in 34 caspase-1 and 11 capase-9 experimentally identified substrates.
Results: This study has made the following findings: first, we verified that caspase-1 and caspase-9 shared 100% aspartic acid in the P1 position. However, the structures in the cleavage sites of most caspase-1 substrates are different from that of caspase-9 substrates in the following three aspects, (a) the amino acid residues with the statistically high frequencies; (b) the hydrophobic amino acid occurrence frequencies; and (c) the charged amino acid occurrence frequencies; second, the amino acid pairs P1-P1' are different; third, our identified cleavage site patterns are useful in the prediction for the 91.4% cleavage sites of 35 new caspase-1 substrates.
Conclusion: Since most caspase-1 substrates are involved in vascular function, inflammation and atherogenesis, our novel structural patterns for the caspases' substrates are significant in developing new diagnostics and therapeutics.
Copyright (c) 2010 Elsevier Ireland Ltd. All rights reserved.
Figures

References
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. - PubMed
-
- Yang XF. Factors regulating apoptosis and homeostasis of CD4+CD25highFOXP3+ regulatory T cells are new therapeutic targets. Front Biosci. 2008;13:1472–1499. - PubMed
-
- Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4:95–104. - PubMed
-
- Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

