Recognition of viruses by cytoplasmic sensors
- PMID: 20061127
- PMCID: PMC3172156
- DOI: 10.1016/j.coi.2009.12.003
Recognition of viruses by cytoplasmic sensors
Abstract
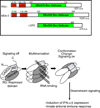
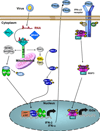
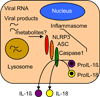
The immune response to virus infection is initiated when pathogen recognition receptors (PRRs) of the host cell recognize specific nonself-motifs within viral products (known as a pathogen-associated molecular pattern or PAMP) to trigger intracellular signaling events that induce innate immunity, the front line of defense against microbial infection. The replication program of all viruses includes a cytosolic phase of genome amplification and/or mRNA metabolism and viral protein expression. Cytosolic recognition of viral infection by specific PRRs takes advantage of the dependence of viruses on the cytosolic component of their replication programs. Such PRR-PAMP interactions lead to PRR-dependent nonself-recognition and the downstream induction of type I interferons and proinflammatory cytokines. These factors serve to induce innate immune programs and drive the maturation of adaptive immunity and inflammation for the control of infection. Recent studies have focused on identifying the particular viral ligands recognized as nonself by cytosolic PRRs, and on defining the nature of the PRRs and their signaling pathways involved in immunity. The RIG-I-like receptors, RIG-I and MDA5, have been defined as essential PRRs for host detection of a variety of RNA viruses. Novel PRRs and their signaling pathways involved in detecting DNA viruses through nonself-recognition of viral DNA are also being elucidated. Moreover, studies to identify the PRRs and signaling factors of the host cell that mediate inflammatory signaling through inflammasome activation following virus infection are currently underway and have already revealed specific NOD-like receptors (NLRs) as inflammatory triggers. This review summarizes recent progress and current areas of focus in pathogen recognition and immune triggering by cytosolic PRRs.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
References
-
- Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65. - PubMed
-
- Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Jr., Akira S, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. - PubMed
-
- Takahasi K, Yoneyama M, Nishihori T, Hirai R, Kumeta H, Narita R, Gale M, Jr., Inagaki F, Fujita T. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell. 2008;29:428–440. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

