Utilization of the 1,2,3,5-thiatriazolidin-3-one 1,1-dioxide scaffold in the design of potential inhibitors of human neutrophil proteinase 3
- PMID: 20061159
- PMCID: PMC2853038
- DOI: 10.1016/j.bmc.2009.12.057
Utilization of the 1,2,3,5-thiatriazolidin-3-one 1,1-dioxide scaffold in the design of potential inhibitors of human neutrophil proteinase 3
Abstract
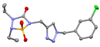
The S' subsites of human neutrophil proteinase 3 (Pr 3) were probed by constructing diverse libraries of compounds based on the 1,2,3,5-thiatriazolidin-3-one 1,1-dioxide using combinational and click chemistry methods. The multiple points of diversity embodied in the heterocyclic scaffold render it well-suited to the exploration of the S' subsites of Pr 3. Molecular modeling studies suggest that further exploration of the S' subsites of Pr 3 using the aforementioned heterocyclic scaffold may lead to the identification of highly selective, reversible competitive inhibitors of Pr 3.
Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Figures
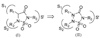



Similar articles
-
Mechanism-based inhibitors of serine proteases with high selectivity through optimization of S' subsite binding.Bioorg Med Chem. 2009 May 15;17(10):3536-42. doi: 10.1016/j.bmc.2009.04.011. Epub 2009 Apr 12. Bioorg Med Chem. 2009. PMID: 19394830 Free PMC article.
-
Inactivation of human neutrophil elastase by 1,2,5-thiadiazolidin-3-one 1,1 dioxide-based sulfonamides.Bioorg Med Chem. 2008 Jan 15;16(2):692-8. doi: 10.1016/j.bmc.2007.10.041. Epub 2007 Oct 18. Bioorg Med Chem. 2008. PMID: 17976994 Free PMC article.
-
Effects of structure on inhibitory activity in a series of mechanism-based inhibitors of human neutrophil elastase.Bioorg Med Chem. 2010 Sep 15;18(18):6646-50. doi: 10.1016/j.bmc.2010.07.071. Epub 2010 Aug 5. Bioorg Med Chem. 2010. PMID: 20728366 Free PMC article.
-
Proteinase 3 phosphonic inhibitors.Biochimie. 2019 Nov;166:142-149. doi: 10.1016/j.biochi.2019.03.005. Epub 2019 Mar 13. Biochimie. 2019. PMID: 30876969 Review.
-
Structures of human proteinase 3 and neutrophil elastase--so similar yet so different.FEBS J. 2010 May;277(10):2238-54. doi: 10.1111/j.1742-4658.2010.07659.x. FEBS J. 2010. PMID: 20423453 Review.
Cited by
-
Design, Synthesis and Cytotoxicity of Thiazole-Based Stilbene Analogs as Novel DNA Topoisomerase IB Inhibitors.Molecules. 2022 Feb 2;27(3):1009. doi: 10.3390/molecules27031009. Molecules. 2022. PMID: 35164276 Free PMC article.
-
An expeditious and efficient bromomethylation of thiols: enabling bromomethyl sulfides as useful building blocks.RSC Adv. 2018 Jul 10;8(43):24654-24659. doi: 10.1039/c8ra04002h. eCollection 2018 Jul 2. RSC Adv. 2018. PMID: 35557855 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

